Enhancement of Peroxidase Stability Against Oxidative Self-Inactivation by Co-immobilization with a Redox-Active Protein in Mesoporous Silicon and Silica Microparticles
- PMID: 27650291
- PMCID: PMC5030200
- DOI: 10.1186/s11671-016-1605-4
Enhancement of Peroxidase Stability Against Oxidative Self-Inactivation by Co-immobilization with a Redox-Active Protein in Mesoporous Silicon and Silica Microparticles
Abstract
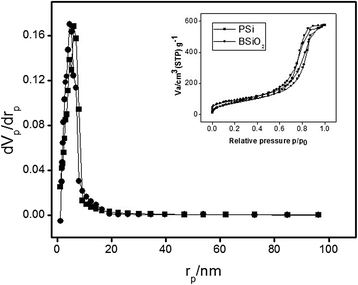
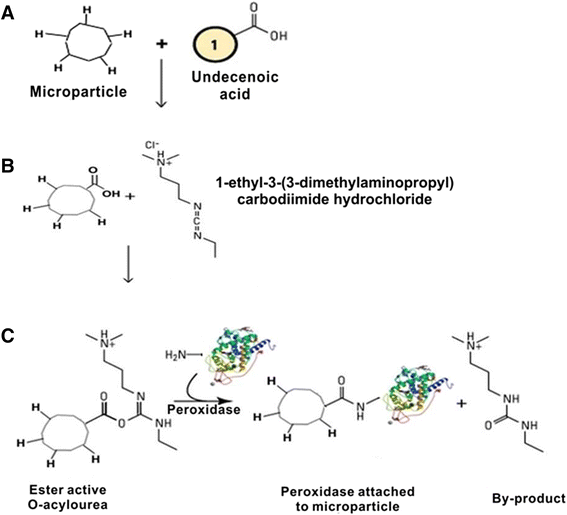
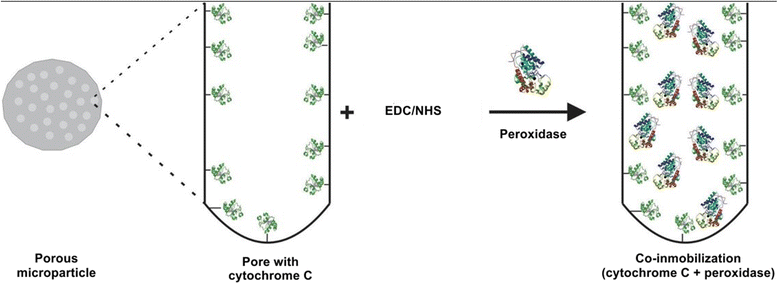
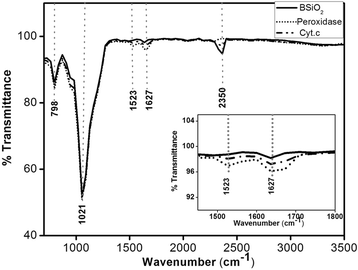
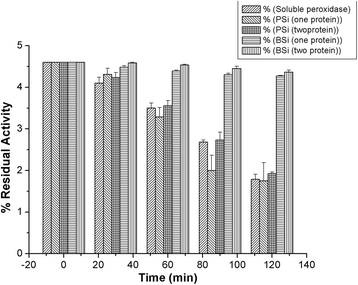
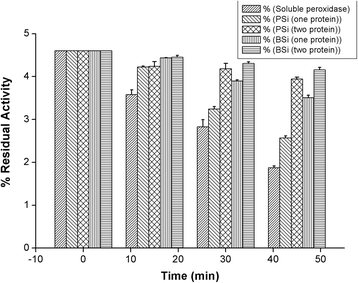
The study of the stability enhancement of a peroxidase immobilized onto mesoporous silicon/silica microparticles is presented. Peroxidases tend to get inactivated in the presence of hydrogen peroxide, their essential co-substrate, following an auto-inactivation mechanism. In order to minimize this inactivation, a second protein was co-immobilized to act as an electron acceptor and thus increase the stability against self-oxidation of peroxidase. Two heme proteins were immobilized into the microparticles: a fungal commercial peroxidase and cytochrome c from equine heart. Two types of biocatalysts were prepared: one with only covalently immobilized peroxidase (one-protein system) and another based on covalent co-immobilization of peroxidase and cytochrome c (two-protein system), both immobilized by using carbodiimide chemistry. The amount of immobilized protein was estimated spectrophotometrically, and the characterization of the biocatalyst support matrix was performed using Brunauer-Emmett-Teller (BET), scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX), and Fourier transform infrared (FTIR) analyses. Stability studies show that co-immobilization with the two-protein system enhances the oxidative stability of peroxidase almost four times with respect to the one-protein system. Thermal stability analysis shows that the immobilization of peroxidase in derivatized porous silicon microparticles does not protect the protein from thermal denaturation, whereas biogenic silica microparticles confer significant thermal stabilization.
Keywords: Auto-inactivation; Microparticles; Peroxidase; Porous silica; Porous silicon.
Figures




References
-
- Bai ZW, Zhou YK. A novel enzyme support derived from aminated silica gel and polysuccinimide: preparation and application for the immobilization of porcine pancreatic lipase. React Funct Polym. 2004;59:93–98. doi: 10.1016/j.reactfunctpolym.2003.12.013. - DOI
-
- Maria P, Chagas B, Torres JA, Silva MC, Nogueira GE, Donizete C, Corrêa AD. Catalytic stability of turnip peroxidase in free and immobilized form on chitosan beads. Int J Curr Microbiol App Sci. 2014;3:576–595.
LinkOut - more resources
Full Text Sources
Other Literature Sources

