Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis
- PMID: 27650485
- PMCID: PMC5035995
- DOI: 10.1038/ncomms12876
Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis
Abstract
Engineering the surface structure at the atomic level can be used to precisely and effectively manipulate the reactivity and durability of catalysts. Here we report tuning of the atomic structure of one-dimensional single-crystal cobalt (II) oxide (CoO) nanorods by creating oxygen vacancies on pyramidal nanofacets. These CoO nanorods exhibit superior catalytic activity and durability towards oxygen reduction/evolution reactions. The combined experimental studies, microscopic and spectroscopic characterization, and density functional theory calculations reveal that the origins of the electrochemical activity of single-crystal CoO nanorods are in the oxygen vacancies that can be readily created on the oxygen-terminated {111} nanofacets, which favourably affect the electronic structure of CoO, assuring a rapid charge transfer and optimal adsorption energies for intermediates of oxygen reduction/evolution reactions. These results show that the surface atomic structure engineering is important for the fabrication of efficient and durable electrocatalysts.
Figures


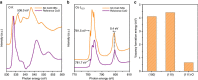
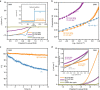

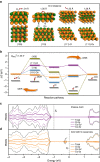
References
-
- Winter M. & Brodd R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4269 (2004). - PubMed
-
- Li Y. et al.. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 4, 1805 (2013). - PubMed
-
- Cheng F. & Chen J. Metal-air batteries: from oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 41, 2172–2192 (2012). - PubMed
-
- Zhang J., Zhao Z., Xia Z. & Dai L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

