The NADPH Oxidases DUOX1 and NOX2 Play Distinct Roles in Redox Regulation of Epidermal Growth Factor Receptor Signaling
- PMID: 27650496
- PMCID: PMC5087744
- DOI: 10.1074/jbc.M116.749028
The NADPH Oxidases DUOX1 and NOX2 Play Distinct Roles in Redox Regulation of Epidermal Growth Factor Receptor Signaling
Abstract
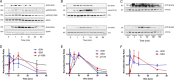
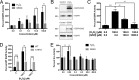
The epidermal growth factor receptor (EGFR) plays a critical role in regulating airway epithelial homeostasis and responses to injury. Activation of EGFR is regulated by redox-dependent processes involving reversible cysteine oxidation by reactive oxygen species (ROS) and involves both ligand-dependent and -independent mechanisms, but the precise source(s) of ROS and the molecular mechanisms that control tyrosine kinase activity are incompletely understood. Here, we demonstrate that stimulation of EGFR activation by ATP in airway epithelial cells is closely associated with dynamic reversible oxidation of cysteine residues via sequential sulfenylation and S-glutathionylation within EGFR and the non-receptor-tyrosine kinase Src. Moreover, the intrinsic kinase activity of recombinant Src or EGFR was in both cases enhanced by H2O2 but not by GSSG, indicating that the intermediate sulfenylation is the activating modification. H2O2-induced increase in EGFR tyrosine kinase activity was not observed with the C797S variant, confirming Cys-797 as the redox-sensitive cysteine residue that regulates kinase activity. Redox-dependent regulation of EGFR activation in airway epithelial cells was found to strongly depend on activation of either the NADPH oxidase DUOX1 or the homolog NOX2, depending on the activation mechanism. Whereas DUOX1 and Src play a primary role in EGFR transactivation by wound-derived signals such as ATP, direct ligand-dependent EGFR activation primarily involves NOX2 with a secondary role for DUOX1 and Src. Collectively, our findings establish that redox-dependent EGFR kinase activation involves a dynamic and reversible cysteine oxidation mechanism and that this activation mechanism variably involves DUOX1 and NOX2.
Keywords: DUOX1; NOX2; Src; cysteine; epidermal growth factor receptor (EGFR); epithelium; hydrogen peroxide; redox signaling; sulfenic acid.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





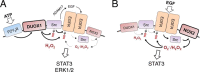
References
-
- Yarden Y., and Schlessinger J. (1987) Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry 26, 1443–1451 - PubMed
-
- Ohtsu H., Dempsey P. J., and Eguchi S. (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 291, C1–C10 - PubMed
-
- Liebmann C. (2011) EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol. Cell. Endocrinol. 331, 222–231 - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

