Fibroblast growth factor receptor 4 induced resistance to radiation therapy in colorectal cancer
- PMID: 27650548
- PMCID: PMC5342528
- DOI: 10.18632/oncotarget.12099
Fibroblast growth factor receptor 4 induced resistance to radiation therapy in colorectal cancer
Erratum in
-
Correction: Fibroblast growth factor receptor 4 induced resistance to radiation therapy in colorectal cancer.Oncotarget. 2019 Sep 3;10(51):5385-5386. doi: 10.18632/oncotarget.27186. eCollection 2019 Sep 3. Oncotarget. 2019. PMID: 31555396 Free PMC article.
Abstract
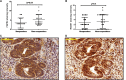
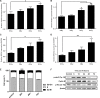
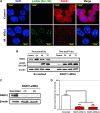
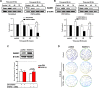
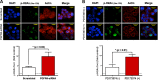
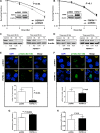
In colorectal cancer (CRC), fibroblast growth factor receptor 4 (FGFR4) is upregulated and acts as an oncogene. This study investigated the impact of this receptor on the response to neoadjuvant radiotherapy by analyzing its levels in rectal tumors of patients with different responses to the therapy. Cellular mechanisms of FGFR4-induced radioresistance were analyzed by silencing or over-expressing FGFR4 in CRC cell line models. Our findings showed that the FGFR4 staining score was significantly higher in pre-treatment biopsies of non-responsive than responsive patients. Similarly, high expression of FGFR4 inhibited radiation response in cell line models. Silencing or inhibition of FGFR4 resulted in a reduction of RAD51 levels and decreased survival in radioresistant HT29 cells. Increased RAD51 expression rescued cells in the siFGFR4-group. In radiosensitive SW480 and DLD1 cells, enforced expression of FGFR4 stabilized RAD51 protein levels resulting in enhanced clearance of γ-H2AX foci and increased cell survival in the mismatch repair (MMR)-proficient SW480 cells. MMR-deficient DLD1 cells are defective in homologous recombination repair and no FGFR4-induced radioresistance was observed. Based on our results, FGFR4 may serve as a predictive marker to select CRC patients with MMR-proficient tumors who may benefit from pre-operative radiotherapy.
Keywords: FGFR4; RAD51; colorectal cancer; radiotherapy.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

