Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline
- PMID: 27650684
- PMCID: PMC5413203
- DOI: 10.1016/j.ygyno.2016.05.022
Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline
Abstract
Purpose: To provide guidance to clinicians regarding the use of neoadjuvant chemotherapy and interval cytoreduction among women with stage IIIC or IV epithelial ovarian cancer.
Methods: The Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an Expert Panel and conducted a systematic review of the literature.
Results: Four phase III clinical trials form the primary evidence base for the recommendations. The published studies suggest that for selected women with stage IIIC or IV epithelial ovarian cancer, neoadjuvant chemotherapy and interval cytoreduction are non-inferior to primary cytoreduction and adjuvant chemotherapy with respect to overall and progression-free survival and are associated with less perioperative morbidity and mortality.
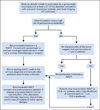
Recommendations: All women with suspected stage IIIC or IV invasive epithelial ovarian cancer should be evaluated by a gynecologic oncologist prior to initiation of therapy. The primary clinical evaluation should include a CT of the abdomen and pelvis, and chest imaging (CT preferred). Women with a high perioperative risk profile or a low likelihood of achieving cytoreduction to <1cm of residual disease (ideally to no visible disease) should receive neoadjuvant chemotherapy. Women who are fit for primary cytoreductive surgery, and with potentially resectable disease, may receive either neoadjuvant chemotherapy or primary cytoreductive surgery. However, primary cytoreductive surgery is preferred if there is a high likelihood of achieving cytoreduction to <1cm (ideally to no visible disease) with acceptable morbidity. Before neoadjuvant chemotherapy is delivered, all patients should have confirmation of an invasive ovarian, fallopian tube, or peritoneal cancer. Additional information is available at www.asco.org/NACT-ovarian-guideline and www.asco.org/guidelineswiki.
Keywords: Chemotherapy; Cytoreductive surgery; Guideline; Neoadjuvant; Ovarian cancer.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures provided by the authors are available with this article at
No relationship to disclose
No relationship to disclose
No relationship to disclose
No relationship to disclose
Figures
References
-
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. - PubMed
-
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. - PubMed
-
- Kang S. Neoadjuvant chemotherapy for ovarian cancer: do we have enough evidence? Lancet. 2015;386:223–224. - PubMed
-
- Fagotti A, Vizzielli G, Fanfani F, et al. Phase III SCORPION trial (ID number: NCT01461850) in epithelial cancer patients with high tumor load receiving PDS versus NACT: An interim analysis on peri-operative outcome; Proceedings Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; 2015. (abstr LBA1)
-
- Onda T, Yoshikawa H, Shibata T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in phase III randomized trial: JCOG0602. J Clin Oncol. 2014;32:353s. (suppl; abstr 5508) - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical