"Threshold-crossing": A Useful Way to Establish the Counterfactual in Clinical Trials?
- PMID: 27650716
- PMCID: PMC5114686
- DOI: 10.1002/cpt.515
"Threshold-crossing": A Useful Way to Establish the Counterfactual in Clinical Trials?
Abstract
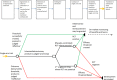
A central question in the assessment of benefit/harm of new treatments is: how does the average outcome on the new treatment (the factual) compare to the average outcome had patients received no treatment or a different treatment known to be effective (the counterfactual)? Randomized controlled trials (RCTs) are the standard for comparing the factual with the counterfactual. Recent developments necessitate and enable a new way of determining the counterfactual for some new medicines. For select situations, we propose a new framework for evidence generation, which we call "threshold-crossing." This framework leverages the wealth of information that is becoming available from completed RCTs and from real world data sources. Relying on formalized procedures, information gleaned from these data is used to estimate the counterfactual, enabling efficacy assessment of new drugs. We propose future (research) activities to enable "threshold-crossing" for carefully selected products and indications in which RCTs are not feasible.
© 2016 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- International Council on Harmonization . ICH Topic E10: Choice of control groups in clinical trials. <http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff...>.
-
- Pocock, S.J. The combination of randomized and historical controls in clinical trials. J. Chronic Dis. 29, 175–188 (1976). - PubMed
-
- Eichler, H.G. et al Bridging the efficacy‐effectiveness gap: a regulator's perspective on addressing variability of drug response. Nat. Rev. Drug Discov. 10, 495–506 (2011). - PubMed
-
- Savović, J. et al Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann. Intern. Med. 157, 429–438 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

