Infectivity of attenuated poxvirus vaccine vectors and immunogenicity of a raccoonpox vectored rabies vaccine in the Brazilian Free-tailed bat (Tadarida brasiliensis)
- PMID: 27650872
- PMCID: PMC5543807
- DOI: 10.1016/j.vaccine.2016.08.088
Infectivity of attenuated poxvirus vaccine vectors and immunogenicity of a raccoonpox vectored rabies vaccine in the Brazilian Free-tailed bat (Tadarida brasiliensis)
Abstract
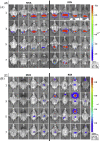
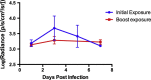
Bats (Order Chiroptera) are an abundant group of mammals with tremendous ecological value as insectivores and plant dispersers, but their role as reservoirs of zoonotic diseases has received more attention in the last decade. With the goal of managing disease in free-ranging bats, we tested modified vaccinia Ankara (MVA) and raccoon poxvirus (RCN) as potential vaccine vectors in the Brazilian Free-tailed bat (Tadarida brasiliensis), using biophotonic in vivo imaging and immunogenicity studies. Animals were administered recombinant poxviral vectors expressing the luciferase gene (MVA-luc, RCN-luc) through oronasal (ON) or intramuscular (IM) routes and subsequently monitored for bioluminescent signal indicative of viral infection. No clinical illness was noted after exposure to any of the vectors, and limited luciferase expression was observed. Higher and longer levels of expression were observed with the RCN-luc construct. When given IM, luciferase expression was limited to the site of injection, while ON exposure led to initial expression in the oral cavity, often followed by secondary replication at another location, likely the gastric mucosa or gastric associated lymphatic tissue. Viral DNA was detected in oral swabs up to 7 and 9 days post infection (dpi) for MVA and RCN, respectively. While no live virus was detected in oral swabs from MVA-infected bats, titers up to 3.88 x 104 PFU/ml were recovered from oral swabs of RCN-infected bats. Viral DNA was also detected in fecal samples from two bats inoculated IM with RCN, but no live virus was recovered. Finally, we examined the immunogenicity of a RCN based rabies vaccine (RCN-G) following ON administration. Significant rabies neutralizing antibody titers were detected in the serum of immunized bats using the rapid fluorescence focus inhibition test (RFFIT). These studies highlight the safety and immunogenicity of attenuated poxviruses and their potential use as vaccine vectors in bats.
Keywords: Bat; Chiroptera; Poxvirus; Rabies; Vaccine.
Published by Elsevier Ltd.
Figures



References
-
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 Oct;310(5):676–679. - PubMed
-
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P. Fruit bats as reservoirs of Ebola virus. Nature. 2005 Dec 1;438(7068):575–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

