Loss of TAB3 expression by shRNA exhibits suppressive bioactivity and increased chemical sensitivity of ovarian cancer cell lines via the NF-κB pathway
- PMID: 27651027
- PMCID: PMC6496425
- DOI: 10.1111/cpr.12293
Loss of TAB3 expression by shRNA exhibits suppressive bioactivity and increased chemical sensitivity of ovarian cancer cell lines via the NF-κB pathway
Abstract
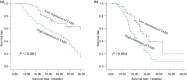
Ovarian cancer is a leading cause of death among gynaecologic malignancies. Despite many years of research, it still remains sparing in reliable diagnostic markers and methods for early detection and screening. Transforming growth factor β-activated protein kinase 1 (TAK1)-binding protein 3 (TAB3) was initially characterized as an adapter protein essential for TAK1 activation in response to IL-1β or TNFα, however, the physiological role of TAB3 in ovarian cancer tumorigenesis is still not fully understood. In this study, we evaluated the effects of TAB3 on ovarian cancer cell lines. Expressions of TAB3 and PCNA (proliferating cell nuclear antigen) were found to be gradually increased in EOC tissues and cell lines, by western blot analysis and qRT-PCR. Distribution of TAB3 was further analysed by immunohistochemistry. In vitro, knockdown of TAB3 expression in HO8910 or SKOV3 ovarian cancer cells significantly inhibited bioactivity of ovarian cancer cells, including proliferation and cell-cycle distribution, and promoted chemical sensitivity to cisplatin and paclitaxel treatment via inhibiting NF-κB pathways. In conclusion, our study strongly suggests a novel function of TAB3 as an oncogene that could be used as a biomarker for ovarian cancer. It provides a new insight into the potential mechanism for therapeutic targeting, in chemotherapy resistance, common in ovarian cancer.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- English DP, Menderes G, Black J, Schwab CL, Santin AD. Molecular diagnosis and molecular profiling to detect treatment‐resistant ovarian cancer. Expert Rev Mol Diagn. 2016;16:769–782. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Mirandola L, et al. Cancer testis antigens: novel biomarkers and targetable proteins for ovarian cancer. Int Rev Immunol. 2011;30:127–137. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

