Heterogeneity among Isolates Reveals that Fitness in Low Oxygen Correlates with Aspergillus fumigatus Virulence
- PMID: 27651366
- PMCID: PMC5040115
- DOI: 10.1128/mBio.01515-16
Heterogeneity among Isolates Reveals that Fitness in Low Oxygen Correlates with Aspergillus fumigatus Virulence
Abstract
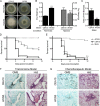
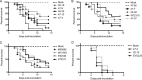
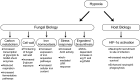
Previous work has shown that environmental and clinical isolates of Aspergillus fumigatus represent a diverse population that occupies a variety of niches, has extensive genetic diversity, and exhibits virulence heterogeneity in a number of animal models of invasive pulmonary aspergillosis (IPA). However, mechanisms explaining differences in virulence among A. fumigatus isolates remain enigmatic. Here, we report a significant difference in virulence of two common lab strains, CEA10 and AF293, in the murine triamcinolone immunosuppression model of IPA, in which we previously identified severe low oxygen microenvironments surrounding fungal lesions. Therefore, we hypothesize that the ability to thrive within these lesions of low oxygen promotes virulence of A. fumigatus in this model. To test this hypothesis, we performed in vitro fitness and in vivo virulence analyses in the triamcinolone murine model of IPA with 14 environmental and clinical isolates of A. fumigatus Among these isolates, we observed a strong correlation between fitness in low oxygen in vitro and virulence. In further support of our hypothesis, experimental evolution of AF293, a strain that exhibits reduced fitness in low oxygen and reduced virulence in the triamcinolone model of IPA, results in a strain (EVOL20) that has increased hypoxia fitness and a corresponding increase in virulence. Thus, the ability to thrive in low oxygen correlates with virulence of A. fumigatus isolates in the context of steroid-mediated murine immunosuppression.
Importance: Aspergillus fumigatus occupies multiple environmental niches, likely contributing to the genotypic and phenotypic heterogeneity among isolates. Despite reports of virulence heterogeneity, pathogenesis studies often utilize a single strain for the identification and characterization of virulence and immunity factors. Here, we describe significant variation between A. fumigatus isolates in hypoxia fitness and virulence, highlighting the advantage of including multiple strains in future studies. We also illustrate that hypoxia fitness correlates strongly with increased virulence exclusively in the nonleukopenic murine triamcinolone immunosuppression model of IPA. Through an experimental evolution experiment, we observe that chronic hypoxia exposure results in increased virulence of A. fumigatus We describe here the first observation of a model-specific virulence phenotype correlative with in vitro fitness in hypoxia and pave the way for identification of hypoxia-mediated mechanisms of virulence in the fungal pathogen A. fumigatus.
Copyright © 2016 Kowalski et al.
Figures



Comment in
-
Heterogeneity Confounds Establishment of "a" Model Microbial Strain.mBio. 2017 Feb 21;8(1):e00135-17. doi: 10.1128/mBio.00135-17. mBio. 2017. PMID: 28223452 Free PMC article.
References
-
- Duarte-Escalante E, Zúñiga G, Ramírez ON, Córdoba S, Refojo N, Arenas R, Delhaes L, Reyes-Montes MDR. 2009. Population structure and diversity of the pathogenic fungus Aspergillus fumigatus isolated from different sources and geographic origins. Mem Inst Oswaldo Cruz 104:427–433. doi:10.1590/S0074-02762009000300005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
