A small stem-loop structure of the Ebola virus trailer is essential for replication and interacts with heat-shock protein A8
- PMID: 27651462
- PMCID: PMC5175359
- DOI: 10.1093/nar/gkw825
A small stem-loop structure of the Ebola virus trailer is essential for replication and interacts with heat-shock protein A8
Abstract
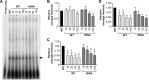
Ebola virus (EBOV) is a single-stranded negative-sense RNA virus belonging to the Filoviridae family. The leader and trailer non-coding regions of the EBOV genome likely regulate its transcription, replication, and progeny genome packaging. We investigated the cis-acting RNA signals involved in RNA-RNA and RNA-protein interactions that regulate replication of eGFP-encoding EBOV minigenomic RNA and identified heat shock cognate protein family A (HSC70) member 8 (HSPA8) as an EBOV trailer-interacting host protein. Mutational analysis of the trailer HSPA8 binding motif revealed that this interaction is essential for EBOV minigenome replication. Selective 2'-hydroxyl acylation analyzed by primer extension analysis of the secondary structure of the EBOV minigenomic RNA indicates formation of a small stem-loop composed of the HSPA8 motif, a 3' stem-loop (nucleotides 1868-1890) that is similar to a previously identified structure in the replicative intermediate (RI) RNA and a panhandle domain involving a trailer-to-leader interaction. Results of minigenome assays and an EBOV reverse genetic system rescue support a role for both the panhandle domain and HSPA8 motif 1 in virus replication.
Published by Oxford University Press on behalf of Nucleic Acids Research 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

Similar articles
-
The Ebola virus genomic replication promoter is bipartite and follows the rule of six.J Virol. 2005 Aug;79(16):10660-71. doi: 10.1128/JVI.79.16.10660-10671.2005. J Virol. 2005. PMID: 16051858 Free PMC article.
-
Modeling Ebola Virus Genome Replication and Transcription with Minigenome Systems.Methods Mol Biol. 2017;1628:79-92. doi: 10.1007/978-1-4939-7116-9_6. Methods Mol Biol. 2017. PMID: 28573612
-
RNA binding specificity of Ebola virus transcription factor VP30.RNA Biol. 2016 Sep;13(9):783-98. doi: 10.1080/15476286.2016.1194160. Epub 2016 Jun 17. RNA Biol. 2016. PMID: 27315567 Free PMC article.
-
Host Factors Involved in Ebola Virus Replication.Curr Top Microbiol Immunol. 2018;419:113-150. doi: 10.1007/82_2017_27. Curr Top Microbiol Immunol. 2018. PMID: 28710692 Review.
-
Computational genomic analysis of hemorrhagic fever viruses. Viral selenoproteins as a potential factor in pathogenesis.Biol Trace Elem Res. 1997 Jan;56(1):93-106. doi: 10.1007/BF02778985. Biol Trace Elem Res. 1997. PMID: 9152513 Review.
Cited by
-
Target Discovery of Matrine against PRRSV in Marc-145 Cells via Activity-Based Protein Profiling.Int J Mol Sci. 2023 Jul 16;24(14):11526. doi: 10.3390/ijms241411526. Int J Mol Sci. 2023. PMID: 37511286 Free PMC article.
-
Development of a Měnglà virus minigenome and comparison of its polymerase complexes with those of other filoviruses.Virol Sin. 2024 Jun;39(3):459-468. doi: 10.1016/j.virs.2024.03.011. Epub 2024 May 21. Virol Sin. 2024. PMID: 38782261 Free PMC article.
-
Staufen1 Interacts with Multiple Components of the Ebola Virus Ribonucleoprotein and Enhances Viral RNA Synthesis.mBio. 2018 Oct 9;9(5):e01771-18. doi: 10.1128/mBio.01771-18. mBio. 2018. PMID: 30301857 Free PMC article.
-
Identification and characterization of short leader and trailer RNAs synthesized by the Ebola virus RNA polymerase.PLoS Pathog. 2021 Oct 26;17(10):e1010002. doi: 10.1371/journal.ppat.1010002. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34699554 Free PMC article.
-
Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development.Signal Transduct Target Ther. 2020 Jul 13;5(1):125. doi: 10.1038/s41392-020-00233-4. Signal Transduct Target Ther. 2020. PMID: 32661235 Free PMC article. Review.
References
-
- Elliott L.H., Sanchez A., Holloway B.P., Kiley M.P., McCormick J.B. Ebola protein analyses for the determination of genetic organization. Arch. Virol. 1993;133:423–436. - PubMed
-
- Sanchez A., Kiley M.P., Holloway B.P., Auperin D.D. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215–240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

