Single-cell dynamics and variability of MAPK activity in a yeast differentiation pathway
- PMID: 27651485
- PMCID: PMC5056058
- DOI: 10.1073/pnas.1610081113
Single-cell dynamics and variability of MAPK activity in a yeast differentiation pathway
Abstract
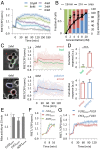
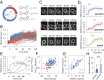
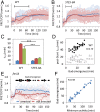
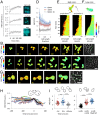
In response to pheromones, yeast cells activate a MAPK pathway to direct processes important for mating, including gene induction, cell-cycle arrest, and polarized cell growth. Although a variety of assays have been able to elucidate signaling activities at multiple steps in the pathway, measurements of MAPK activity during the pheromone response have remained elusive, and our understanding of single-cell signaling behavior is incomplete. Using a yeast-optimized FRET-based mammalian Erk-activity reporter to monitor Fus3 and Kss1 activity in live yeast cells, we demonstrate that overall mating MAPK activity exhibits distinct temporal dynamics, rapid reversibility, and a graded dose dependence around the KD of the receptor, where phenotypic transitions occur. The complex dose response was found to be largely a consequence of two feedbacks involving cyclin-mediated scaffold phosphorylation and Fus3 autoregulation. Distinct cell cycle-dependent response patterns comprised a large portion of the cell-to-cell variability at each dose, constituting the major source of extrinsic noise in coupling activity to downstream gene-expression responses. Additionally, we found diverse spatial MAPK activity patterns to emerge over time in cells undergoing default, gradient, and true mating responses. Furthermore, ramping up and rapid loss of activity were closely associated with zygote formation in mating-cell pairs, supporting a role for elevated MAPK activity in successful cell fusion and morphogenic reorganization. Altogether, these findings present a detailed view of spatiotemporal MAPK activity during the pheromone response, elucidating its role in mediating complex long-term developmental fates in a unicellular differentiation system.
Keywords: Fus3; MAPK dynamics; cell signaling; mating pathway; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mitogen-activated protein kinase (MAPK) dynamics determine cell fate in the yeast mating response.J Biol Chem. 2017 Dec 15;292(50):20354-20361. doi: 10.1074/jbc.AC117.000548. Epub 2017 Nov 9. J Biol Chem. 2017. PMID: 29123025 Free PMC article.
-
MAPK specificity in the yeast pheromone response independent of transcriptional activation.Curr Biol. 2001 Aug 21;11(16):1266-71. doi: 10.1016/s0960-9822(01)00370-0. Curr Biol. 2001. PMID: 11525741
-
Fus3-regulated Tec1 degradation through SCFCdc4 determines MAPK signaling specificity during mating in yeast.Cell. 2004 Dec 29;119(7):981-90. doi: 10.1016/j.cell.2004.11.053. Cell. 2004. PMID: 15620356
-
Spatial and temporal signal processing and decision making by MAPK pathways.J Cell Biol. 2017 Feb;216(2):317-330. doi: 10.1083/jcb.201609124. Epub 2017 Jan 2. J Cell Biol. 2017. PMID: 28043970 Free PMC article. Review.
-
Dynamics and organization of MAP kinase signal pathways.Mol Reprod Dev. 1995 Dec;42(4):477-85. doi: 10.1002/mrd.1080420416. Mol Reprod Dev. 1995. PMID: 8607979 Review.
Cited by
-
Global quantitative understanding of non-equilibrium cell fate decision-making in response to pheromone.iScience. 2023 Sep 9;26(10):107885. doi: 10.1016/j.isci.2023.107885. eCollection 2023 Oct 20. iScience. 2023. PMID: 37766979 Free PMC article.
-
Gain- and loss-of-function alleles within signaling pathways lead to phenotypic diversity among individuals.iScience. 2024 Aug 31;27(10):110860. doi: 10.1016/j.isci.2024.110860. eCollection 2024 Oct 18. iScience. 2024. PMID: 39381740 Free PMC article.
-
Orientation of Cell Polarity by Chemical Gradients.Annu Rev Biophys. 2022 May 9;51:431-451. doi: 10.1146/annurev-biophys-110821-071250. Epub 2022 Feb 7. Annu Rev Biophys. 2022. PMID: 35130037 Free PMC article. Review.
-
Interaction of AURKA with TRIM28 revives dormant LSCC cells via Akt signaling pathway to promote LSCC metastasis.Cancer Cell Int. 2025 Jan 3;25(1):2. doi: 10.1186/s12935-024-03620-x. Cancer Cell Int. 2025. PMID: 39754170 Free PMC article.
-
Spatial modeling of the membrane-cytosolic interface in protein kinase signal transduction.PLoS Comput Biol. 2018 Apr 9;14(4):e1006075. doi: 10.1371/journal.pcbi.1006075. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29630597 Free PMC article.
References
-
- Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2004;25(9):1465–1476. - PubMed
-
- Butty A-C, Pryciak PM, Huang LS, Herskowitz I, Peter M. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science. 1998;282(5393):1511–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

