A Retrospective Case-Control Study Evaluating the Role of Mifepristone for Induction of Labor in Women with Previous Cesarean Section
- PMID: 27651574
- PMCID: PMC5016401
- DOI: 10.1007/s13224-015-0760-3
A Retrospective Case-Control Study Evaluating the Role of Mifepristone for Induction of Labor in Women with Previous Cesarean Section
Abstract
Objective: To investigate the role of "mifepristone" for induction of labor (IOL) in pregnant women with prior cesarean section (CS).
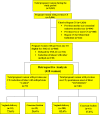
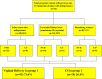
Methods: In this retrospective study, all pregnant women with prior CS who received oral mifepristone (400 mg) for IOL (as per clear obstetric indications) [group 1] were compared with pregnant women with prior CS who had spontaneous onset of labor (SOL) [group 2], with respect to incidence of vaginal delivery, CS, duration of labor, and various maternal and fetal outcomes.
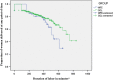
Results: During the study period, 72 women received mifepristone (group 1) for IOL and 346 had SOL (group 2). In group 1 after mifepristone administration, 40 (55.6 %) women had labor onset, and 24 (33.3 %) women had cervical ripening (Bishop Score ≥ 8) within 48 h. There were no statistically significant differences with respect to duration of labor (p value: 0.681), mode of delivery (i.e., normal delivery or CS-p value: 0.076 or 0.120, respectively), or maternal (blood loss or scar dehiscence/rupture uterus), or fetal outcomes (NICU admission) compared to women with previous CS with SOL (group 2). However, the need of oxytocin (p value 0.020) and dose of oxytocin requirement (p value 0.008) were more statistically significant in group 1.
Conclusion: Mifepristone may be considered as an agent for IOL in women with prior CS.
Keywords: Induction of labor; Mifepristone; Pregnancy; Prior cesarean delivery.
Conflict of interest statement
There is no conflict of interest with any individual or organization. Ethical Statement Compliance with ethical requirements and Conflict of interest Ethical clearance has been taken from ethical committee of Dr. RPGMC Kangra at Tanda (HFW-H-DRPGMC/Ethics/2014) keeping in view the ICMR guidelines (1994) and Helinski declaration (modified 2000). Written informed consent was taken from all the women. Identity of every women is kept as secret. All the women were adequately informed of the aims, methods, and any discomfort it may entail to her and the remedies thereof. Every precaution was taken to respect the privacy of the patient, the confidentiality of the patient’s information, and to minimize the impact of the study on her physical and mental integrity and her personality. The patient was given the right to abstain from the study or to withdraw consent to participate at any time of study without reappraisal.
Figures
References
-
- ACOG Practice bulletin no. 115 Vaginal birth after previous cesarean delivery. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2010;116(4):450–463. - PubMed
-
- Jozwiak M, Dodd JM. Methods of term labour induction for women with a previous caesarean section. Cochrane Database Syst Rev. 2013 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
