Carboprost Versus Oxytocin for Active Management of Third Stage of Labor: A Prospective Randomized Control Study
- PMID: 27651609
- PMCID: PMC5016447
- DOI: 10.1007/s13224-016-0842-x
Carboprost Versus Oxytocin for Active Management of Third Stage of Labor: A Prospective Randomized Control Study
Abstract
Background and objectives: Postpartum hemorrhage is the single largest and leading cause of maternal morbidity and mortality not only in developing countries but also in developed countries. The present study is an attempt to evaluate the scope of using prophylactic intramuscular carboprost tromethamine 125 μg in comparison with intramuscular oxytocin 10 units for the active management of third stage of labor.
Materials and methods: Two hundred pregnant women at term with spontaneous onset of labor were included in the study and were randomly divided into 2 groups of 100 women each. Group A and group B were given injection oxytocin 10 units and injection carboprost tromethamine 125 μg intramuscularly, respectively, at the time of delivery of anterior shoulder. The main outcome measures with respect to third stage of labor were: duration, blood loss by volume, difference in hemoglobin, need for additional oxytocics and side effects.
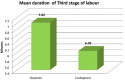
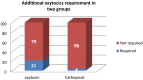
Results: Subjects who received carboprost tromethamine 125 μg showed a significant reduction in duration of third stage of labor (p < 0.05) and blood loss (p < 0.01) when compared to the subjects who received oxytocin 10 units. Likelihood of occurrence of postpartum hemorrhage was reduced without significant side effects except for diarrhea. Additional need for other uterotonics after carboprost was significantly less compared to oxytocin.
Conclusion: Intramuscular carboprost 125 μg is a better cost-effective alternative as compared to 10 units intramuscular oxytocin in active management of third stage of labor.
Keywords: Active management of third stage of labor; Brasss V drape; Carboprost; Oxytocin; Postpartum hemorrhage; Third stage of labor.
Conflict of interest statement
Compliance with Ethical Statement Conflict of interest All authors of the study declare that there is no conflict of interest of any type. Ethical Statement Permission was obtained from the ethical committee of the institution.
Figures
References
-
- Shah D, Divakar H, Meghal T. Combating post partum hemorrhage in India: moving forward. In: Christopher B-L, Louis GK, Andre BL, Mahantesh K, editors. A Textbook of postpartum hemorrhage, a comprehensive guide to evaluate, management and surgical intervention. 1. UK: Sapiens Publishing; 2006. pp. 434–441.
-
- Park K. Preventive medicine in obstetrics, pediatrics and geriatrics. In: Park K, editor. Textbook of preventive and social medicine. 22. Banasridas Bhanot: Jabalpur; 2013. pp. 516–519.
-
- Bhattacharya P, Devi PK, Jain S, et al. Prophylactic use of 15(S) 15 methyl PGF2 alpha by intramuscular route for control of postpartum bleeding—a comparative trial with methylergometrine. Acta Obstet Gynecol Scand. 1988;145 Suppl:S13–S15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources