Role of Intravenous Tranexamic Acid on Caesarean Blood Loss: A Prospective Randomised Study
- PMID: 27651628
- PMCID: PMC5016480
- DOI: 10.1007/s13224-016-0915-x
Role of Intravenous Tranexamic Acid on Caesarean Blood Loss: A Prospective Randomised Study
Abstract
Background: Post-partum haemorrhage (PPH) is a major cause of maternal mortality globally. Tranexamic acid, an anti-fibrinolytic agent, is a novel approach in an attempt to prevent this dreadful complication. This study aims to document the efficacy of intravenous (IV) tranexamic acid in reducing blood loss during and after caesarean section (CS).
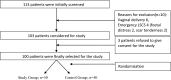
Methods: In this prospective randomised placebo-controlled open-label study, 100 mothers scheduled for elective CS were randomly selected and divided into two groups (study and control) of 50 each. The study group received 1 g IV tranexamic acid and the control group received IV placebo. Following delivery, all mothers received ten units of oxytocin in 500 ml of normal saline.
Results: The mean intra-operative and post-partum blood loss were significantly lower in the study group than the control group: 499.11 ± 111.2 and 59.93 ± 12.5 ml versus 690.85 ± 198.41 and 110.06 ± 13.47 ml, respectively, (p < 0.001). Total blood loss was 30 % less in the study group (p < 0.001). Six mothers had PPH in the control group, while none in the study group. The difference between the pre-operative and post-operative haemoglobin levels was significantly less in the study group than the control group, 0.26 ± 0.22 versus 0.99 ± 0.48 g% (p < 0.001).There was no significant difference with respect to other haematological parameters. There was no added adverse effect or need for NICU admission in the study group.
Conclusion: Pre-operative IV tranexamic acid significantly reduced blood loss during elective CS without any significant adverse effects.
Keywords: Anti-fibrinolytics; Blood loss; Caesarean delivery; Post-partum haemorrhage; Tranexamic acid.
Conflict of interest statement
The authors declare that they have no conflicts of interest. Research involving human participants and/or animals No animals were involved in this study. Only human participants were included in both case and control. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed Consent Written informed consent has been taken from all patients participating in the trial.
References
-
- AbouZahr C. Antepartum and postpartum haemorrhage. Chapter 4. First edition. Boston, Geneva: Harvard School of Public Health on behalf of the World Health Organisation and the World Bank; 1998.
-
- Combs CA, Murphy EL, LarosJr RK. Factors associated with haemorrhage in cesarean deliveries. Obstet Gynecol. 1991;77(1):77–82. - PubMed
-
- Munn MB, Owen J, Vincent R, et al. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2001;98:386–390. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical