Improved cartilage regeneration by implantation of acellular biomaterials after bone marrow stimulation: a systematic review and meta-analysis of animal studies
- PMID: 27651981
- PMCID: PMC5018675
- DOI: 10.7717/peerj.2243
Improved cartilage regeneration by implantation of acellular biomaterials after bone marrow stimulation: a systematic review and meta-analysis of animal studies
Abstract
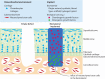
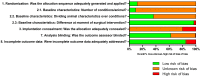
Microfracture surgery may be applied to treat cartilage defects. During the procedure the subchondral bone is penetrated, allowing bone marrow-derived mesenchymal stem cells to migrate towards the defect site and form new cartilage tissue. Microfracture surgery generally results in the formation of mechanically inferior fibrocartilage. As a result, this technique offers only temporary clinical improvement. Tissue engineering and regenerative medicine may improve the outcome of microfracture surgery. Filling the subchondral defect with a biomaterial may provide a template for the formation of new hyaline cartilage tissue. In this study, a systematic review and meta-analysis were performed to assess the current evidence for the efficacy of cartilage regeneration in preclinical models using acellular biomaterials implanted after marrow stimulating techniques (microfracturing and subchondral drilling) compared to the natural healing response of defects. The review aims to provide new insights into the most effective biomaterials, to provide an overview of currently existing knowledge, and to identify potential lacunae in current studies to direct future research. A comprehensive search was systematically performed in PubMed and EMBASE (via OvidSP) using search terms related to tissue engineering, cartilage and animals. Primary studies in which acellular biomaterials were implanted in osteochondral defects in the knee or ankle joint in healthy animals were included and study characteristics tabulated (283 studies out of 6,688 studies found). For studies comparing non-treated empty defects to defects containing implanted biomaterials and using semi-quantitative histology as outcome measure, the risk of bias (135 studies) was assessed and outcome data were collected for meta-analysis (151 studies). Random-effects meta-analyses were performed, using cartilage regeneration as outcome measure on an absolute 0-100% scale. Implantation of acellular biomaterials significantly improved cartilage regeneration by 15.6% compared to non-treated empty defect controls. The addition of biologics to biomaterials significantly improved cartilage regeneration by 7.6% compared to control biomaterials. No significant differences were found between biomaterials from natural or synthetic origin or between scaffolds, hydrogels and blends. No noticeable differences were found in outcome between animal models. The risk of bias assessment indicated poor reporting for the majority of studies, impeding an assessment of the actual risk of bias. In conclusion, implantation of biomaterials in osteochondral defects improves cartilage regeneration compared to natural healing, which is further improved by the incorporation of biologics.
Keywords: Biomaterials; Cartilage; Cell-free; Microfracture; Osteochondral; Regenerative medicine; Scaffold.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Athanasiou K, Korvick D, Schenck Jr R. Biodegradable implants for the treatment of osteochondral defects in a goat model. Tissue Engineering. 1997;3:363–373. doi: 10.1089/ten.1997.3.363. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

