Niclosamide suppresses renal cell carcinoma by inhibiting Wnt/β-catenin and inducing mitochondrial dysfunctions
- PMID: 27652012
- PMCID: PMC5005241
- DOI: 10.1186/s40064-016-3153-x
Niclosamide suppresses renal cell carcinoma by inhibiting Wnt/β-catenin and inducing mitochondrial dysfunctions
Abstract
Purpose: To investigate the effects of anthelminthic drug niclosamide in renal cell carcinoma (RCC) and the underlying mechanisms of its action.
Methods: The effects of niclosamide on the proliferation and apoptosis of RCC cells were examined in vitro and in vivo by using MTS, colony formation assay, flow cytometry and xenograft cancer mouse model. Mechanism studies were performed by analyzing Wnt/β-catenin signaling and mitochondrial functions in a panel of RCC cell lines.
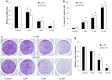
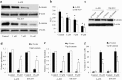
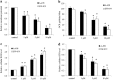
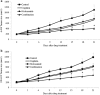
Results: We show that niclosamide effectively targets two RCC cell lines through inhibiting proliferation and anchorage-independent colony formation, and inducing apoptosis. It also enhances the inhibitory effects of chemotherapeutic drug cisplatin in two independent in vivo RCC xenograft mouse models. Mechanistically, niclosamide decreases β-catenin levels and therefore suppresses Wnt/β-catenin activities. Overexpression of β-catenin partially reverses the inhibitory effects of niclosamide in RCC cells, demonstrating that besides β-catenin, other mechanisms are involved in niclosamide's anti-cancer activity. Indeed, we further show that niclosamide induces mitochondrial dysfunctions as shown by the decreased level of mitochondrial membrane potential and respiration, resulting in decreased ATP levels and increased reactive oxygen species (ROS) levels.
Conclusions: Our findings support the inhibitory effects of niclosamide in cancer and provide better understanding on its underlying mechanism. Our data suggests that niclosamide is a useful addition to the treatment armamentarium for RCC.
Keywords: Mitochondria; Niclosamide; Renal cell carcinoma; Wnt/β-catenin.
Figures
Similar articles
-
Pyrvinium Sensitizes Clear Cell Renal Cell Carcinoma Response to Chemotherapy Via Casein Kinase 1α-Dependent Inhibition of Wnt/β-Catenin.Am J Med Sci. 2018 Mar;355(3):274-280. doi: 10.1016/j.amjms.2017.11.017. Epub 2017 Dec 2. Am J Med Sci. 2018. PMID: 29549930
-
Benzimidazole inhibitors from the Niclosamide chemotype inhibit Wnt/β-catenin signaling with selectivity over effects on ATP homeostasis.Bioorg Med Chem. 2017 Mar 15;25(6):1804-1816. doi: 10.1016/j.bmc.2017.01.046. Epub 2017 Feb 3. Bioorg Med Chem. 2017. PMID: 28233680 Free PMC article.
-
Inhibition of Wnt/β-catenin by anthelmintic drug niclosamide effectively targets growth, survival, and angiogenesis of retinoblastoma.Am J Transl Res. 2017 Aug 15;9(8):3776-3786. eCollection 2017. Am J Transl Res. 2017. PMID: 28861168 Free PMC article.
-
Targeting Wnt/β-catenin by anthelmintic drug niclosamide overcomes paclitaxel resistance in esophageal cancer.Fundam Clin Pharmacol. 2021 Feb;35(1):165-173. doi: 10.1111/fcp.12583. Epub 2020 Jul 13. Fundam Clin Pharmacol. 2021. PMID: 32579788
-
Niclosamide: A career builder.J Control Release. 2024 May;369:786-856. doi: 10.1016/j.jconrel.2023.07.016. Epub 2024 Mar 21. J Control Release. 2024. PMID: 37544514 Review.
Cited by
-
Targeting Reactive Oxygen Species Capacity of Tumor Cells with Repurposed Drug as an Anticancer Therapy.Oxid Med Cell Longev. 2021 Sep 7;2021:8532940. doi: 10.1155/2021/8532940. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34539975 Free PMC article. Review.
-
The anthelmintic drug niclosamide induces GSK-β-mediated β-catenin degradation to potentiate gemcitabine activity, reduce immune evasion ability and suppress pancreatic cancer progression.Cell Death Dis. 2022 Feb 3;13(2):112. doi: 10.1038/s41419-022-04573-7. Cell Death Dis. 2022. PMID: 35115509 Free PMC article.
-
Involvement of Up-Regulation of DR5 Expression and Down-Regulation of c-FLIP in Niclosamide-Mediated TRAIL Sensitization in Human Renal Carcinoma Caki Cells.Molecules. 2018 Sep 5;23(9):2264. doi: 10.3390/molecules23092264. Molecules. 2018. PMID: 30189637 Free PMC article.
-
Niclosamide Revitalizes Sorafenib through Insulin-like Growth Factor 1 Receptor (IGF-1R)/Stemness and Metabolic Changes in Hepatocellular Carcinoma.Cancers (Basel). 2023 Feb 1;15(3):931. doi: 10.3390/cancers15030931. Cancers (Basel). 2023. PMID: 36765890 Free PMC article.
-
Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori.Sci Rep. 2018 Feb 27;8(1):3701. doi: 10.1038/s41598-018-22037-x. Sci Rep. 2018. PMID: 29487357 Free PMC article.
References
-
- Cojocaru E, Lozneanu L, Giusca SE, Caruntu ID, Danciu M. Renal carcinogenesis—insights into signaling pathways. Rom J Morphol Embryol = Revue roumaine de morphologie et embryologie. 2015;56(1):15–19. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

