Daclatasvir combined with peginterferon-α and ribavirin for the treatment of chronic hepatitis C: a meta-analysis
- PMID: 27652142
- PMCID: PMC5023653
- DOI: 10.1186/s40064-016-3218-x
Daclatasvir combined with peginterferon-α and ribavirin for the treatment of chronic hepatitis C: a meta-analysis
Abstract
Daclatasvir, a HCV NS5A inhibitor, is a new direct-acting antiviral drug for chronic hepatitis C (CHC). This study aimed to evaluate the efficacy and safety of daclatasvir combined with peginterferon-α (pegIFN-α) and ribavirin (RBV) for the treatment of CHC. The databases of PUBMED, EMBASE, COCHRANE, WANFANG, and CNKI were retrieved to identify eligible studies. Pooled risk ratio (RR) and 95 % confidence interval (CI) were calculated using random or fixed models. A total of six RCTs including 1100 adult patients with CHC met the inclusion criteria and the patients were infected with HCV genotype 1-4, with the genotype 1 infection accounting for 73.1 %. Meta-analysis showed daclatasvir-based combination therapy yielded a significantly higher probability of achieving the overall RVR (46.43 vs. 18.97 %) with pooled RR of 3.77 (95 % CI 1.95-7.28, p < 0.0001) and a slightly higher probability of achieving the overall SVR24 (65.08 vs. 47.77 %) with pooled RR of 1.41 (95 % CI 1.18-1.68, p < 0.0001), and did not show increased adverse events compared with the pegIFN-α/RBV regimen (control group). Subgroup analysis showed the rate of RVR and SVR24 in high-dose daclatasvir (60 mg/day) group were slightly higher than the overall results; the rate of RVR in low-dose daclatasvir (10 mg/day) group was also higher than the control group, but its SVR24 rate was similar between the two groups. Daclatasvir combined with pegIFN-α/RBV is effective and safe in treating adult patients with CHC, especially HCV genotype 1 infection, and daclatasvir (60 mg/day) is a better choice as compared with daclatasvir (10 mg/day).
Keywords: Chronic; Daclatasvir; Hepatitis C; Meta-analysis; Randomized controlled trials.
Figures







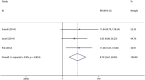
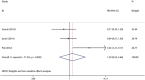
References
-
- AASLD/IDSA/IAS–USA (2014) Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed 11 Aug 2014
-
- American Association for the Study of the Liver (2016) Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/fullreport. Accessed 11 Aug 2016
-
- Centers for Disease Control and Prevention (2014) Hepatitis C FAQs for healthcare professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section1. Accessed 1 Aug 2014
-
- Chen GF. The problems and challenges of the treatment of chronic hepatitis C in DAAs era. J Med Res. 2016;45:1–7.
-
- Chen GF, Li B. The progress and prospect of treatment of the hepatitis C virus infection. J Med Res. 2015;44:1–17.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

