The design and methodology of premature ejaculation interventional studies
- PMID: 27652224
- PMCID: PMC5002005
- DOI: 10.21037/tau.2016.03.28
The design and methodology of premature ejaculation interventional studies
Abstract
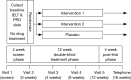
Large well-designed clinical efficacy and safety randomized clinical trials (RCTs) are required to achieve regulatory approval of new drug treatments. The objective of this article is to make recommendations for the criteria for defining and selecting the clinical trial study population, design and efficacy outcomes measures which comprise ideal premature ejaculation (PE) interventional trial methodology. Data on clinical trial design, epidemiology, definitions, dimensions and psychological impact of PE was reviewed, critiqued and incorporated into a series of recommendations for standardisation of PE clinical trial design, outcome measures and reporting using the principles of evidence based medicine. Data from PE interventional studies are only reliable, interpretable and capable of being generalised to patients with PE, when study populations are defined by the International Society for Sexual Medicine (ISSM) multivariate definition of PE. PE intervention trials should employ a double-blind RCT methodology and include placebo control, active standard drug control, and/or dose comparison trials. Ejaculatory latency time (ELT) and subject/partner outcome measures of control, personal/partner/relationship distress and other study-specific outcome measures should be used as outcome measures. There is currently no published literature which identifies a clinically significant threshold response to intervention. The ISSM definition of PE reflects the contemporary understanding of PE and represents the state-of-the-art multi-dimensional definition of PE and is recommended as the basis of diagnosis of PE for all PE clinical trials.
Keywords: Treatment; premature ejaculation (PE); trial methodology.
Conflict of interest statement
Dr. Mcmahon is or has been a consultant, investigator and speaker for Johnson & Johnson, Janssen Cilag, Menarini, Ixchelsis, Absorption Pharmaceuticals, NeuroHealing and Plethora.
Figures
Similar articles
-
Clinical trial methodology in premature ejaculation observational, interventional, and treatment preference studies--part II--study design, outcome measures, data analysis, and reporting.J Sex Med. 2008 Aug;5(8):1817-33. doi: 10.1111/j.1743-6109.2008.00837.x. Epub 2008 May 26. J Sex Med. 2008. PMID: 18507716 Review.
-
Clinical trial methodology in premature ejaculation observational, interventional, and treatment preference studies--part I--defining and selecting the study population.J Sex Med. 2008 Aug;5(8):1805-16. doi: 10.1111/j.1743-6109.2008.00836.x. Epub 2008 May 26. J Sex Med. 2008. PMID: 18507717 Review.
-
An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation.J Sex Med. 2008 Jul;5(7):1590-606. doi: 10.1111/j.1743-6109.2008.00901.x. J Sex Med. 2008. PMID: 18466262
-
An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation.J Sex Med. 2014 Jun;11(6):1423-41. doi: 10.1111/jsm.12524. Epub 2014 May 22. J Sex Med. 2014. PMID: 24848805 Review.
-
An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation.Sex Med. 2014 Jun;2(2):41-59. doi: 10.1002/sm2.27. Sex Med. 2014. PMID: 25356301 Free PMC article. Review.
Cited by
-
Efficacy of Sphincter Control Training and medical device in the treatment of premature ejaculation: A multicenter randomized controlled clinical trial.PLoS One. 2021 Sep 21;16(9):e0257284. doi: 10.1371/journal.pone.0257284. eCollection 2021. PLoS One. 2021. PMID: 34547013 Free PMC article. Clinical Trial.
-
Efficacy of Sphincter Control Training (SCT) in the treatment of premature ejaculation, a new cognitive behavioral approach: A parallel-group randomized, controlled trial.PLoS One. 2019 Feb 26;14(2):e0212274. doi: 10.1371/journal.pone.0212274. eCollection 2019. PLoS One. 2019. PMID: 30807583 Free PMC article. Clinical Trial.
-
Transcutaneous electric nerve stimulation to treat patients with premature ejaculation: phase II clinical trial.Int J Impot Res. 2020 Jul;32(4):434-439. doi: 10.1038/s41443-019-0196-x. Epub 2019 Sep 24. Int J Impot Res. 2020. PMID: 31551577 Clinical Trial.
-
Premature ejaculation: challenging new and the old concepts.F1000Res. 2017 Dec 4;6:2084. doi: 10.12688/f1000research.12150.1. eCollection 2017. F1000Res. 2017. PMID: 29259775 Free PMC article. Review.
-
A Comparative Study of Yoga with Paroxetine for the Treatment of Premature Ejaculation: A Pilot Study.Int J Yoga. 2020 Sep-Dec;13(3):227-232. doi: 10.4103/ijoy.IJOY_89_19. Epub 2020 Sep 13. Int J Yoga. 2020. PMID: 33343153 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources