Involvement of Histone Lysine Methylation in p21 Gene Expression in Rat Kidney In Vivo and Rat Mesangial Cells In Vitro under Diabetic Conditions
- PMID: 27652271
- PMCID: PMC5019898
- DOI: 10.1155/2016/3853242
Involvement of Histone Lysine Methylation in p21 Gene Expression in Rat Kidney In Vivo and Rat Mesangial Cells In Vitro under Diabetic Conditions
Abstract
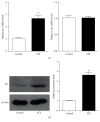
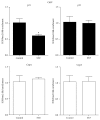
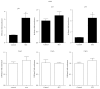
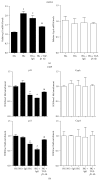
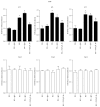
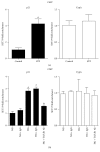
Diabetic nephropathy (DN), a common complication associated with type 1 and type 2 diabetes mellitus (DM), characterized by glomerular mesangial expansion, inflammation, accumulation of extracellular matrix (ECM) protein, and hypertrophy, is the major cause of end-stage renal disease (ESRD). Increasing evidence suggested that p21-dependent glomerular and mesangial cell (MC) hypertrophy play key roles in the pathogenesis of DN. Recently, posttranscriptional modifications (PTMs) have uncovered novel molecular mechanisms involved in DN. However, precise regulatory mechanism of histone lysine methylation (HKme) mediating p21 related hypertrophy associated with DN is not clear. We evaluated the roles of HKme and histone methyltransferase (HMT) SET7/9 in p21 gene expression in glomeruli of diabetic rats and in high glucose- (HG-) treated rat mesangial cells (RMCs). p21 gene expression was upregulated in diabetic rats glomeruli; chromatin immunoprecipitation (ChIP) assays showed decreased histone H3-lysine9-dimethylation (H3K9me2) accompanied with enhanced histone H3-lysine4-methylation (H3K4me1/3) and SET7/9 occupancies at the p21 promoter. HG-treated RMCs exhibited increased p21 mRNA, H3K4me level, SET7/9 recruitment, and inverse H3K9me, which were reversed by TGF-β1 antibody. These data uncovered key roles of H3Kme and SET7/9 responsible for p21 gene expression in vivo and in vitro under diabetic conditions and confirmed preventive effect of TGF-β1 antibody on DN.
Figures
References
-
- Wolf G. Molecular mechanisms of diabetic mesangial cell hypertrophy: a proliferation of novel factors. Journal of the American Society of Nephrology. 2002;13(10):2611–2613. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

