Efficacy and biodistribution analysis of intracerebroventricular administration of an optimized scAAV9-SMN1 vector in a mouse model of spinal muscular atrophy
- PMID: 27652289
- PMCID: PMC5022869
- DOI: 10.1038/mtm.2016.60
Efficacy and biodistribution analysis of intracerebroventricular administration of an optimized scAAV9-SMN1 vector in a mouse model of spinal muscular atrophy
Abstract
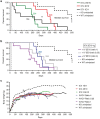
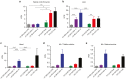
Spinal muscular atrophy (SMA) is an autosomal recessive disease of variable severity caused by mutations in the SMN1 gene. Deficiency of the ubiquitous SMN function results in spinal cord α-motor neuron degeneration and proximal muscle weakness. Gene replacement therapy with recombinant adeno-associated viral (AAV) vectors showed therapeutic efficacy in several animal models of SMA. Here, we report a study aimed at analyzing the efficacy and biodistribution of a serotype-9, self-complementary AAV vector expressing a codon-optimized human SMN1 coding sequence (coSMN1) under the control of the constitutive phosphoglycerate kinase (PGK) promoter in neonatal SMNΔ7 mice, a severe animal model of the disease. We administered the scAAV9-coSMN1 vector in the intracerebroventricular (ICV) space in a dose-escalating mode, and analyzed survival, vector biodistribution and SMN protein expression in the spinal cord and peripheral tissues. All treated mice showed a significant, dose-dependent rescue of lifespan and growth with a median survival of 346 days. Additional administration of vector by an intravenous route (ICV+IV) did not improve survival, and vector biodistribution analysis 90 days postinjection indicated that diffusion from the cerebrospinal fluid to the periphery was sufficient to rescue the SMA phenotype. These results support the preclinical development of SMN1 gene therapy by CSF vector delivery.
Figures

References
-
- Crawford, TO and Pardo, CA (1996). The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis 3: 97–110. - PubMed
-
- Prior, TW, Snyder, PJ, Rink, BD, Pearl, DK, Pyatt, RE, Mihal, DC et al. (2010). Newborn and carrier screening for spinal muscular atrophy. Am J Med Genet A 152A: 1608–1616. - PubMed
-
- Melki, J, Abdelhak, S, Sheth, P, Bachelot, MF, Burlet, P, Marcadet, A et al. (1990). Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 344: 767–768. - PubMed
-
- Lefebvre, S, Bürglen, L, Reboullet, S, Clermont, O, Burlet, P, Viollet, L et al. (1995). Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80: 155–165. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

