Bioorthogonal Strategy for Bioprocessing of Specific-Site-Functionalized Enveloped Influenza-Virus-Like Particles
- PMID: 27652605
- PMCID: PMC5080633
- DOI: 10.1021/acs.bioconjchem.6b00372
Bioorthogonal Strategy for Bioprocessing of Specific-Site-Functionalized Enveloped Influenza-Virus-Like Particles
Abstract
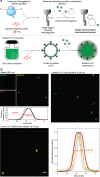
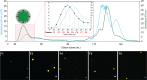
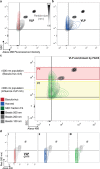
Virus-like particles (VLPs) constitute a promising platform in vaccine development and targeted drug delivery. To date, most applications use simple nonenveloped VLPs as human papillomavirus or hepatitis B vaccines, even though the envelope is known to be critical to retain the native protein folding and biological function. Here, we present tagged enveloped VLPs (TagE-VLPs) as a valuable strategy for the downstream processing and monitoring of the in vivo production of specific-site-functionalized enveloped influenza VLPs. This two-step procedure allows bioorthogonal functionalization of azide-tagged nascent influenza type A hemagglutinin proteins in the envelope of VLPs through a strain-promoted [3 + 2] alkyne-azide cycloaddition reaction. Importantly, labeling does not influence VLP production and allows for construction of functionalized VLPs without deleterious effects on their biological function. Refined discrimination and separation between VLP and baculovirus, the major impurity of the process, is achieved when this technique is combined with flow cytometry analysis, as demonstrated by atomic force microscopy. TagE-VLPs is a versatile tool broadly applicable to the production, monitoring, and purification of functionalized enveloped VLPs for vaccine design trial runs, targeted drug delivery, and molecular imaging.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Production and characterization of mammalian virus-like particles from modified vaccinia virus Ankara vectors expressing influenza H5N1 hemagglutinin and neuraminidase.Vaccine. 2012 May 14;30(23):3413-22. doi: 10.1016/j.vaccine.2012.03.033. Epub 2012 Mar 29. Vaccine. 2012. PMID: 22465746
-
Surface functionalization of virus-like particles via bioorthogonal click reactions for enhanced cell-specific targeting.Int J Pharm. 2024 Jul 20;660:124332. doi: 10.1016/j.ijpharm.2024.124332. Epub 2024 Jun 10. Int J Pharm. 2024. PMID: 38866085
-
VLP-factory™ and ADDomer© : Self-assembling Virus-Like Particle (VLP) Technologies for Multiple Protein and Peptide Epitope Display.Curr Protoc. 2021 Mar;1(3):e55. doi: 10.1002/cpz1.55. Curr Protoc. 2021. PMID: 33729713 Free PMC article.
-
Influenza vaccines based on virus-like particles.Virus Res. 2009 Aug;143(2):140-6. doi: 10.1016/j.virusres.2009.04.005. Epub 2009 Apr 15. Virus Res. 2009. PMID: 19374929 Free PMC article. Review.
-
Plant-derived virus-like particles as vaccines.Hum Vaccin Immunother. 2013 Jan;9(1):26-49. doi: 10.4161/hv.22218. Epub 2012 Sep 20. Hum Vaccin Immunother. 2013. PMID: 22995837 Free PMC article. Review.
Cited by
-
Purification of influenza virus-like particles using sulfated cellulose membrane adsorbers.J Chem Technol Biotechnol. 2018 Jul;93(7):1988-1996. doi: 10.1002/jctb.5474. Epub 2017 Dec 16. J Chem Technol Biotechnol. 2018. PMID: 30008506 Free PMC article.
-
Production of norovirus-, rotavirus-, and enterovirus-like particles in insect cells is simplified by plasmid-based expression.Sci Rep. 2024 Jun 27;14(1):14874. doi: 10.1038/s41598-024-65316-6. Sci Rep. 2024. PMID: 38937523 Free PMC article.
-
An Overview of Recent Developments in the Application of Antigen Displaying Vaccine Platforms: Hints for Future SARS-CoV-2 VLP Vaccines.Vaccines (Basel). 2023 Sep 20;11(9):1506. doi: 10.3390/vaccines11091506. Vaccines (Basel). 2023. PMID: 37766182 Free PMC article. Review.
-
Bioanalytics for Influenza Virus-Like Particle Characterization and Process Monitoring.Front Bioeng Biotechnol. 2022 Feb 18;10:805176. doi: 10.3389/fbioe.2022.805176. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252128 Free PMC article.
-
Improving Influenza HA-Vlps Production in Insect High Five Cells via Adaptive Laboratory Evolution.Vaccines (Basel). 2020 Oct 7;8(4):589. doi: 10.3390/vaccines8040589. Vaccines (Basel). 2020. PMID: 33036359 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

