Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate
- PMID: 27652886
- PMCID: PMC5031981
- DOI: 10.1038/srep33835
Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate
Abstract
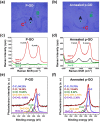
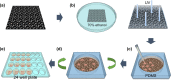
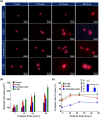
Control of living cells on biocompatible materials or on modified substrates is important for the development of bio-applications, including biosensors and implant biomaterials. The topography and hydrophobicity of substrates highly affect cell adhesion, growth, and cell growth kinetics, which is of great importance in bio-applications. Herein, we investigate the adhesion, growth, and morphology of cultured breast cancer cells on a silicon substrate, on which graphene oxides (GO) was partially formed. By minimizing the size and amount of the GO-containing solution and the further annealing process, GO-coated Si samples were prepared which partially covered the Si substrates. The coverage of GO on Si samples decreases upon annealing. The behaviors of cells cultured on two samples have been observed, i.e. partially GO-coated Si (P-GO) and annealed partially GO-coated Si (Annealed p-GO), with a different coverage of GO. Indeed, the spreading area covered by the cells and the number of cells for a given culture period in the incubator were highly dependent on the hydrophobicity and the presence of oxygenated groups on GO and Si substrates, suggesting hydrophobicity-driven cell growth. Thus, the presented method can be used to control the cell growth via an appropriate surface modification.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

