The putative flavin carrier family FlcA-C is important for Aspergillus fumigatus virulence
- PMID: 27652896
- PMCID: PMC5626198
- DOI: 10.1080/21505594.2016.1239010
The putative flavin carrier family FlcA-C is important for Aspergillus fumigatus virulence
Abstract
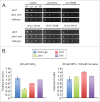
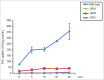
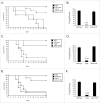
Aspergillus fumigatus is an opportunistic fungal pathogen and the most important species causing pulmonary fungal infections. The signaling by calcium is very important for A. fumigatus pathogenicity and it is regulated by the transcription factor CrzA. We have previously used used ChIP-seq (Chromatin Immunoprecipitation DNA sequencing) aiming to identify gene targets regulated by CrzA. We have identified among several genes regulated by calcium stress, the putative flavin transporter, flcA. This transporter belongs to a small protein family composed of FlcA, B, and C. The ΔflcA null mutant showed several phenotypes, such as morphological defects, increased sensitivity to calcium chelating-agent ethylene glycol tetraacetic acid (EGTA), cell wall or oxidative damaging agents and metals, repre-sentative of deficiencies in calcium signaling and iron homeostasis. Increasing calcium concentrations improved significantly the ΔflcA growth and conidiation, indicating that ΔflcA mutant has calcium insufficiency. Finally, ΔflcA-C mutants showed reduced flavin adenine dinucleotide (FAD) and were avirulent in a low dose murine infection model.
Keywords: Aspergillus fumigatus; CrzA; FAD metabolism; calcium; putative flavin flcA-C transporter family.
Figures





Comment in
-
Balancing iron and calcium: Flavin carrier family proteins in Aspergillus fumigatus virulence.Virulence. 2017 Aug 18;8(6):621-624. doi: 10.1080/21505594.2016.1257459. Epub 2016 Nov 7. Virulence. 2017. PMID: 27820672 Free PMC article. No abstract available.
References
-
- Thewes S. Calcineurin-Crz1 Signaling in Lower Eukaryotes. Eukaryot Cell 2014; 13:694-705; PMID:24681686; http://dx.doi.org/10.1128/EC.00038-14 - DOI - PMC - PubMed
-
- Juvvadi PR, Lee SC, Heitman J, Steinbach WJ. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2016; 20:1-12; http://dx.doi.org/10.1080/21505594.2016.1201250 - DOI - PMC - PubMed
-
- Cyert MS. Calcineurin signaling in Saccharomyces cereviside: how yeast go crazy in response to stress. Biochem Biophys Res Commun 2003; 311:1143-50; PMID:14623300; http://dx.doi.org/10.1016/S0006-291X(03)01552-3 - DOI - PubMed
-
- Steinbach WJ, Reedy JL, Cramer RA Jr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 2007; 5:418-30; PMID:17505522; http://dx.doi.org/10.1038/nrmicro1680 - DOI - PubMed
-
- Stie J, Fox D. Calcineurin regulation in fungi and beyond. Eukaryotic Cell 2008; 7:177-86; PMID:18065652; http://dx.doi.org/10.1128/EC.00326-07 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
