CLINICAL ENDPOINTS FOR THE STUDY OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION
- PMID: 27652913
- PMCID: PMC5384792
- DOI: 10.1097/IAE.0000000000001283
CLINICAL ENDPOINTS FOR THE STUDY OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION
Abstract
Purpose: To summarize the recent literature describing the application of modern technologies in the study of patients with geographic atrophy (GA) secondary to age-related macular degeneration.
Methods: Review of the literature describing the terms and definitions used to describe GA, imaging modalities used to capture and measure GA, and the tests of visual function and functional deficits that occur in patients with GA.
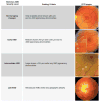
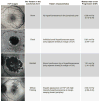
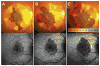
Results: In this paper, we describe the evolution of the definitions used to describe GA. We compare imaging modalities used in the characterization of GA, report on the sensitivity and specificity of the techniques where data exist, and describe the correlations between these various modes of capturing the presence of GA. We review the functional tests that have been used in patients with GA, and critically examine their ability to detect and quantify visual deficits.
Conclusion: Ophthalmologists and retina specialists now have a wide range of assessments available for the functional and anatomic characterization of GA in patients with age-related macular degeneration. To date, studies have been limited by their unimodal approach, and we recommend that future studies of GA use multimodal imaging. We also suggest strategies for the optimal functional testing of patients with GA.
Conflict of interest statement
SriniVas Sadda has served as a consultant to Genentech, Roche, Allergan, Novartis, Stem Cell Inc, Iconic, Avalanche, Optos, and Carl Zeiss Meditec. Usha Chakravarthy has attended advisory boards for Hoffmann-La Roche and Genentech. Her institution has received grants from Hoffmann-La Roche and she is principal investigator in trials sponsored by Roche. David G. Birch has been a consultant for AGTC, Acucela, Inc., Shire Pharmaceuticals, ISIS/GSK, QLT, and Thrombogenics. Giovanni Staurenghi is principal investigator in trials sponsored by Roche. He is also consultant for Heidelberg Engineering, Quantel Medical, Carl Zeiss Meditec, Alcon, Allergan, Bayer, Boheringer, Genentech, GSK, Novartis, Roche. His institution received grants from Optos, Optovue, Centervue, Heidelberg Engineering, Quantel Medical, Novartis, Carl Zeiss Meditec, Alcon, allergan. He has a patent with Ocular Instruments. Erin Henry is an employee of Genentech, Inc. Christopher Brittain is an employee of F. Hoffmann-La Roche, Ltd.
Figures


References
-
- Sunness JS. The natural history of geographic atrophy, the advanced form of age-related macular degeneration. Mol Vis. 1999;5:25. - PubMed
-
- Rudnicka AR, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. - PubMed
-
- Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. - PubMed
-
- Rudnicka AR, et al. Incidence of Late-Stage Age-Related Macular Degeneration in American Whites: Systematic Review and Meta-analysis. American journal of ophthalmology. 2015;160:85–93. e83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

