Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice
- PMID: 27653046
- PMCID: PMC5031985
- DOI: 10.1038/srep33841
Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice
Abstract
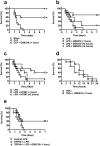
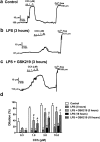
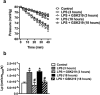
Sepsis is characterized by systemic inflammation, edema formation and hypo-perfusion leading to organ dysfunction and ultimately death. Activation of the transient receptor potential vanilloid type 4 (TRPV4) channel is associated with edema formation and circulatory collapse. Here, we show that TRPV4 channels are involved in the hyper-inflammatory response and mortality associated with sepsis. Pharmacological inhibition of TRPV4 channels in mice reduced mortality in lipopolysaccharide and cecal-ligation-and-puncture models of sepsis, but not in a tumor necrosis factor-α (TNFα)-induced sepsis model. These protective effects of TRPV4 channel inhibition were attributable to prevention of the sepsis-induced surge of a broad spectrum of pro-inflammatory cytokines, including TNFα, interleukin (IL)-1 and IL-6, and subsequent preservation of endothelial cell function, including Ca2+ signaling, integrity and endothelium-dependent vasodilation. These results suggest that TRPV4 antagonists may be of therapeutic utility in the management of sepsis.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

