The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes
- PMID: 27653219
- PMCID: PMC5065312
- DOI: 10.7554/eLife.17716
The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes
Abstract
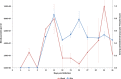
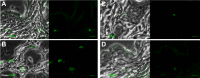
The role of mammalian skin in harbouring and transmitting arthropod-borne protozoan parasites has been overlooked for decades as these pathogens have been regarded primarily as blood-dwelling organisms. Intriguingly, infections with low or undetected blood parasites are common, particularly in the case of Human African Trypanosomiasis caused by Trypanosoma brucei gambiense. We hypothesise, therefore, the skin represents an anatomic reservoir of infection. Here we definitively show that substantial quantities of trypanosomes exist within the skin following experimental infection, which can be transmitted to the tsetse vector, even in the absence of detectable parasitaemia. Importantly, we demonstrate the presence of extravascular parasites in human skin biopsies from undiagnosed individuals. The identification of this novel reservoir requires a re-evaluation of current diagnostic methods and control policies. More broadly, our results indicate that transmission is a key evolutionary force driving parasite extravasation that could further result in tissue invasion-dependent pathology.
Keywords: Human African Trypanosomiasis; Trypanosoma brucei gambiense; epidemiology; global health; human; infectious disease; microbiology; mouse; reservoir; skin; transmission; trypanosomes.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







Comment in
-
Skin deep.Elife. 2016 Oct 14;5:e21506. doi: 10.7554/eLife.21506. Elife. 2016. PMID: 27740910 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

