A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques
- PMID: 27653379
- PMCID: PMC5036149
- DOI: 10.1038/ncomms12838
A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques
Abstract
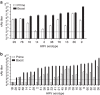
As the predominant aetiological agent of the common cold, human rhinovirus (HRV) is the leading cause of human infectious disease. Early studies showed that a monovalent formalin-inactivated HRV vaccine can be protective, and virus-neutralizing antibodies (nAb) correlated with protection. However, co-circulation of many HRV types discouraged further vaccine efforts. Here, we test the hypothesis that increasing virus input titres in polyvalent inactivated HRV vaccine may result in broad nAb responses. We show that serum nAb against many rhinovirus types can be induced by polyvalent, inactivated HRVs plus alhydrogel (alum) adjuvant. Using formulations up to 25-valent in mice and 50-valent in rhesus macaques, HRV vaccine immunogenicity was related to sufficient quantity of input antigens, and valency was not a major factor for potency or breadth of the response. Thus, we have generated a vaccine capable of inducing nAb responses to numerous and diverse HRV types.
Conflict of interest statement
M.L.M. co-founded and serves as Chief Scientific Officer for Meissa Vaccines, Inc. S.L., M.T.N. and M.L.M are co-inventors in a patent application (PCT/US2016/037658) describing the rhinovirus vaccine reported in this paper. The vaccine technology has been optioned to Meissa Vaccines, Inc. by Emory University. The remaining authors declare no competing financial interests.
Figures



References
-
- Gwaltney J. M. Jr, Hendley J. O., Simon G. & Jordan W. S. Jr Rhinovirus infections in an industrial population. I. The occurrence of illness. N. Engl. J. Med. 275, 1261–1268 (1966). - PubMed
-
- Glanville N. & Johnston S. L. Challenges in developing a cross-serotype rhinovirus vaccine. Curr. Opin. Virol. 11, 83–88 (2015). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

