The Biophysical Basis Underlying Gating Changes in the p.V1316A Mutant Nav1.7 Channel and the Molecular Pathogenesis of Inherited Erythromelalgia
- PMID: 27653502
- PMCID: PMC5031448
- DOI: 10.1371/journal.pbio.1002561
The Biophysical Basis Underlying Gating Changes in the p.V1316A Mutant Nav1.7 Channel and the Molecular Pathogenesis of Inherited Erythromelalgia
Abstract
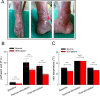
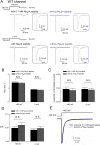
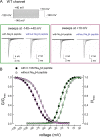
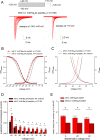
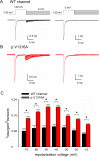
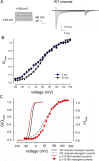
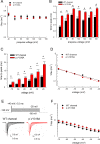
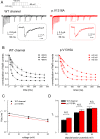
The Nav1.7 channel critically contributes to the excitability of sensory neurons, and gain-of-function mutations of this channel have been shown to cause inherited erythromelalgia (IEM) with neuropathic pain. In this study, we report a case of a severe phenotype of IEM caused by p.V1316A mutation in the Nav1.7 channel. Mechanistically, we first demonstrate that the Navβ4 peptide acts as a gating modifier rather than an open channel blocker competing with the inactivating peptide to give rise to resurgent currents in the Nav1.7 channel. Moreover, there are two distinct open and two corresponding fast inactivated states in the genesis of resurgent Na+ currents. One is responsible for the resurgent route and practically existent only in the presence of Navβ4 peptide, whereas the other is responsible for the "silent" route of recovery from inactivation. In this regard, the p.V1316A mutation makes hyperpolarization shift in the activation curve, and depolarization shift in the inactivation curve, vividly uncoupling inactivation from activation. In terms of molecular gating operation, the most important changes caused by the p.V1316A mutation are both acceleration of the transition from the inactivated states to the activated states and deceleration of the reverse transition, resulting in much larger sustained as well as resurgent Na+ currents. In summary, the genesis of the resurgent currents in the Nav1.7 channel is ascribable to the transient existence of a distinct and novel open state promoted by the Navβ4 peptide. In addition, S4-5 linker in domain III where V1316 is located seems to play a critical role in activation-inactivation coupling, chiefly via direct modulation of the transitional kinetics between the open and the inactivated states. The sustained and resurgent Na+ currents may therefore be correlatively enhanced by specific mutations involving this linker and relevant regions, and thus marked hyperexcitability in corresponding neural tissues as well as IEM symptomatology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

