A computational lens for sexual-stage transmission, reproduction, fitness and kinetics in Plasmodium falciparum
- PMID: 27653663
- PMCID: PMC5031309
- DOI: 10.1186/s12936-016-1538-5
A computational lens for sexual-stage transmission, reproduction, fitness and kinetics in Plasmodium falciparum
Abstract
Background: The burden of falciparum malaria remains unacceptably high in much of sub-Saharan Africa and massive efforts are underway to eliminate the parasite. While symptoms of malaria are caused by asexual reproduction of the parasite, transmission to new human hosts relies entirely on male and female sexual-stage parasites, known as gametocytes. Successful transmission can be observed at very low gametocyte densities, which raises the question of whether transmission-enhancing mechanisms exist in the human host, the mosquito, or both.
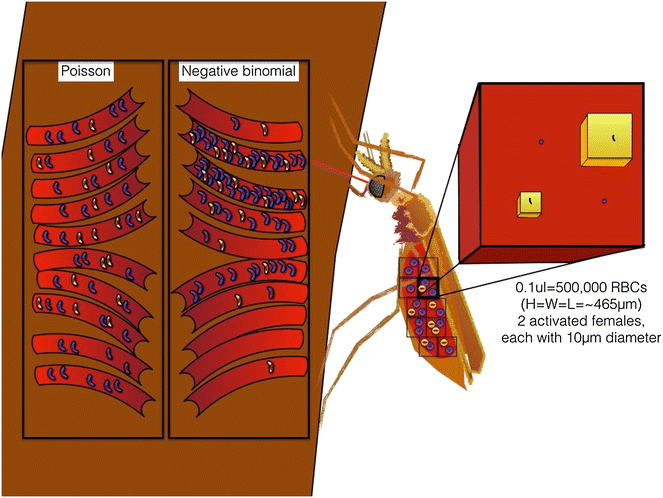
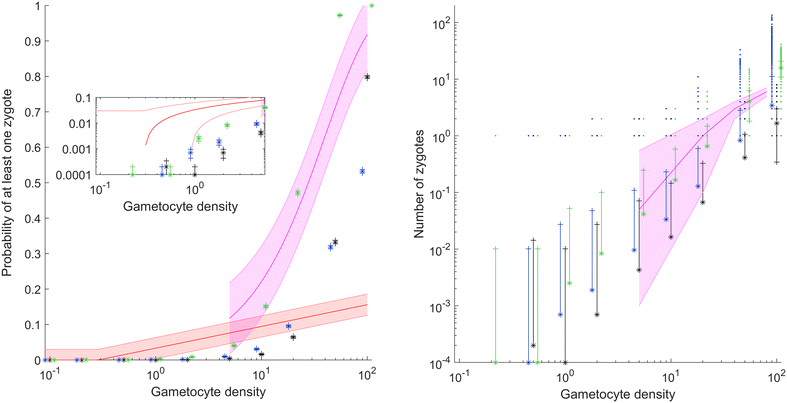
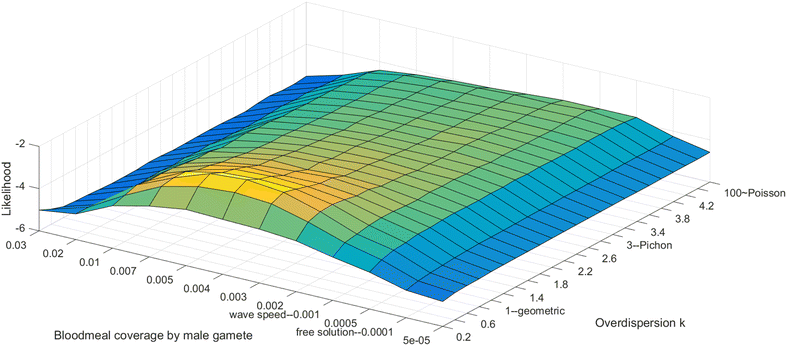
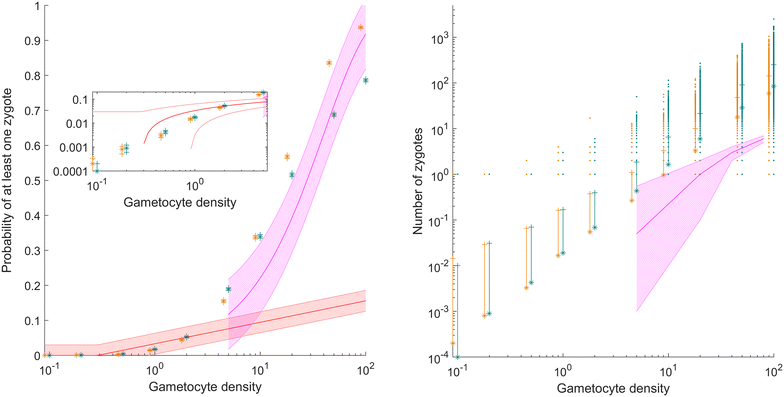
Methods: A new computational model was developed to investigate the probability of fertilization over a range of overdispersion parameters and male gamete exploration rates. Simulations were used to fit a likelihood surface for data on rates of mosquito infection across a wide range of host gametocyte densities.
Results: The best fit simultaneously requires very strong overdispersion and faster gamete exploration than is possible with random swimming in order to explain typical prevalence levels in mosquitoes. Gametocyte overdispersion or clustering in the human host and faster gamete exploration of the mosquito blood meal are highly probably given these results.
Conclusions: Density-dependent gametocyte clustering in the human host, and non-random searching (e.g., chemotaxis) in the mosquito are probable. Future work should aim to discover these mechanisms, as disrupting parasite development in the mosquito will play a critical role in eliminating malaria.
Keywords: Gametocytes; Mathematical model; Plasmodium falciparum.
Figures
References
-
- Anderson RM, May RM. Regulation and stability of host-parasite population interactions. J Anim Ecol. 1978;47:219–247. doi: 10.2307/3933. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

