Direct C(sp3)-H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals
- PMID: 27653738
- PMCID: PMC5215658
- DOI: 10.1021/jacs.6b08397
Direct C(sp3)-H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals
Abstract
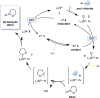
Here we report the development of a C(sp3)-H cross-coupling platform enabled by the catalytic generation of chlorine radicals by nickel and photoredox catalysis. Aryl chlorides serve as both cross-coupling partners and the chlorine radical source for the α-oxy C(sp3)-H arylation of cyclic and acyclic ethers. Mechanistic studies suggest that photolysis of a Ni(III) aryl chloride intermediate, generated by photoredox-mediated single-electron oxidation, leads to elimination of a chlorine radical in what amounts to the sequential capture of two photons. Arylations of a benzylic C(sp3)-H bond of toluene and a completely unactivated C(sp3)-H bond of cyclohexane demonstrate the broad implications of this manifold for accomplishing numerous C(sp3)-H bond functionalizations under exceptionally mild conditions.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures



References
-
- Fokin AA, Schreiner PR. Chem Rev. 2002;102:1551–1594. - PubMed
-
- Carey FA, Sundberg RJ. Advanced Organic Chemistry Part A: Structure and Mechanisms. Springer; New York: 2007. pp. 965–1063.
-
- Ananikov VP. ACS Catal. 2015;5:1964–1971.
-
- Tellis JC, Primer DN, Molander GA. Science. 2014;345:433–436. - PMC - PubMed
- Weix DJ. Acc Chem Res. 2015;48:1767–1775. - PMC - PubMed
- Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan C, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. J Am Chem Soc. 2016;138:2174–2177. - PMC - PubMed
- Shaw MH, Twilton J, MacMillan DWC. J Org Chem. 2016;81:6898–6926. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

