Ciprofloxacin Exposure Leading to Fatal Hepatotoxicity: An Unusual Correlation
- PMID: 27653941
- PMCID: PMC5036381
- DOI: 10.12659/ajcr.899080
Ciprofloxacin Exposure Leading to Fatal Hepatotoxicity: An Unusual Correlation
Abstract
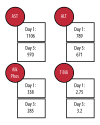
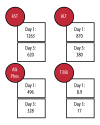
BACKGROUND Ciprofloxacin is a commonly used fluoroquinolone antibiotic. It is occasionally associated with benign elevations in liver enzymes. Few reports in the literature correlate ciprofloxacin with significant liver injury. We present a fatal case of ciprofloxacin-induced liver failure. CASE REPORT A 74-year-old female was successfully treated with ciprofloxacin for a urinary tract infection (UTI), but immediately began having new-onset symptoms, including fatigue and nausea. This continued for two months, at which time she presented to the hospital; she was found to have elevated liver enzymes and another UTI. She was treated with ciprofloxacin again for UTI and discharged three days later, following mild improvement. One week later, she returned to another hospital and was found to have more significantly elevated liver function tests and jaundice. Extensive viral and autoimmune panels were unremarkable. Liver biopsy showed cholestatic hepatitis of unclear etiology. The patient was discharged again following a mild decline in liver enzymes. Soon after, the patient was admitted to our institution with similar complaints. Serum transaminases remained elevated, with an increase in alkaline phosphatase and bilirubin. The Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scale was found to be 8, outlining a high or definite probability that the ciprofloxacin was the cause of the patient's hepatotoxicity. A one-week course of prednisone for possible hypersensitivity reaction was tried; however, it proved unsuccessful. Palliative care was consulted, and the patient passed away shortly thereafter. CONCLUSIONS This case demonstrates the potential for liver failure from ciprofloxacin. Clinicians should evaluate the possibility of ciprofloxacin-induced hepatotoxicity in a patient presenting with liver injury of unknown etiology. Similarly, it is important to consider this significant effect when a practitioner considers antibiotic choice.
Figures

References
-
- NIH National Institutes of Health and LiverTox [Internet] Bethesda (MD): Ciprofloxacin; [(accessed on 29 January 2015)]. Available from: URL: http://livertox.nlm.nih.gov/Ciprofloxacin.htm.
-
- Fuchs S, Simon Z, Brezis M. Fatal hepatic failure associated with ciprofloxacin. Lancet. 1994;343:738–39. - PubMed
-
- Grassmick BK, Lehr VT, Sundareson AS. Fulminant hepatic failure possibly related to ciprofloxacin. Ann Pharmacother. 1992;26:636–39. - PubMed
LinkOut - more resources
Full Text Sources

