PrPC Governs Susceptibility to Prion Strains in Bank Vole, While Other Host Factors Modulate Strain Features
- PMID: 27654300
- PMCID: PMC5110189
- DOI: 10.1128/JVI.01592-16
PrPC Governs Susceptibility to Prion Strains in Bank Vole, While Other Host Factors Modulate Strain Features
Abstract
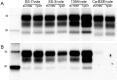
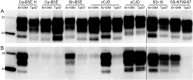
Bank vole is a rodent species that shows differential susceptibility to the experimental transmission of different prion strains. In this work, the transmission features of a panel of diverse prions with distinct origins were assayed both in bank vole expressing methionine at codon 109 (Bv109M) and in transgenic mice expressing physiological levels of bank vole PrPC (the BvPrP-Tg407 mouse line). This work is the first systematic comparison of the transmission features of a collection of prion isolates, representing a panel of diverse prion strains, in a transgenic-mouse model and in its natural counterpart. The results showed very similar transmission properties in both the natural species and the transgenic-mouse model, demonstrating the key role of the PrP amino acid sequence in prion transmission susceptibility. However, differences in the PrPSc types propagated by Bv109M and BvPrP-Tg407 suggest that host factors other than PrPC modulate prion strain features.
Importance: The differential susceptibility of bank voles to prion strains can be modeled in transgenic mice, suggesting that this selective susceptibility is controlled by the vole PrP sequence alone rather than by other species-specific factors. Differences in the phenotypes observed after prion transmissions in bank voles and in the transgenic mice suggest that host factors other than the PrPC sequence may affect the selection of the substrain replicating in the animal model.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
A domain responsible for spontaneous conversion of bank vole prion protein.Brain Pathol. 2019 Mar;29(2):155-163. doi: 10.1111/bpa.12638. Epub 2018 Oct 29. Brain Pathol. 2019. PMID: 30051525 Free PMC article.
-
Spontaneous generation of rapidly transmissible prions in transgenic mice expressing wild-type bank vole prion protein.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3498-503. doi: 10.1073/pnas.1121556109. Epub 2012 Feb 13. Proc Natl Acad Sci U S A. 2012. PMID: 22331873 Free PMC article.
-
Assessment of the PrPc Amino-Terminal Domain in Prion Species Barriers.J Virol. 2016 Nov 14;90(23):10752-10761. doi: 10.1128/JVI.01121-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654299 Free PMC article.
-
The utility of bank voles for studying prion disease.Prog Mol Biol Transl Sci. 2020;175:179-211. doi: 10.1016/bs.pmbts.2020.08.009. Epub 2020 Sep 8. Prog Mol Biol Transl Sci. 2020. PMID: 32958232 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
-
PMCA-generated prions from the olfactory mucosa of patients with Fatal Familial Insomnia cause prion disease in mice.Elife. 2021 Apr 14;10:e65311. doi: 10.7554/eLife.65311. Elife. 2021. PMID: 33851575 Free PMC article.
-
Cofactor molecules: Essential partners for infectious prions.Prog Mol Biol Transl Sci. 2020;175:53-75. doi: 10.1016/bs.pmbts.2020.07.009. Epub 2020 Aug 24. Prog Mol Biol Transl Sci. 2020. PMID: 32958241 Free PMC article. Review.
-
Classical BSE dismissed as the cause of CWD in Norwegian red deer despite strain similarities between both prion agents.Vet Res. 2024 May 15;55(1):62. doi: 10.1186/s13567-024-01320-y. Vet Res. 2024. PMID: 38750594 Free PMC article.
-
Characterization of goat prions demonstrates geographical variation of scrapie strains in Europe and reveals the composite nature of prion strains.Sci Rep. 2020 Jan 8;10(1):19. doi: 10.1038/s41598-019-57005-6. Sci Rep. 2020. PMID: 31913327 Free PMC article.
-
A single amino acid residue in bank vole prion protein drives permissiveness to Nor98/atypical scrapie and the emergence of multiple strain variants.PLoS Pathog. 2022 Jun 22;18(6):e1010646. doi: 10.1371/journal.ppat.1010646. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35731839 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

