A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein
- PMID: 27654301
- PMCID: PMC5110185
- DOI: 10.1128/JVI.01253-16
A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein
Abstract
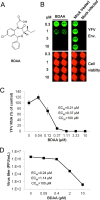
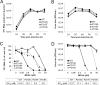
Although a highly effective vaccine is available, the number of yellow fever cases has increased over the past 2 decades, which highlights the pressing need for antiviral therapeutics. In a high-throughput screening campaign, we identified an acetic acid benzodiazepine (BDAA) compound which potently inhibits yellow fever virus (YFV). Interestingly, while treatment of YFV-infected cultures with 2 μM BDAA reduced the virion production by greater than 2 logs, the compound was not active against 21 other viruses from 14 different viral families. Selection and genetic analysis of drug-resistant viruses revealed that replacement of the proline at amino acid 219 (P219) of the nonstructural protein 4B (NS4B) with serine, threonine, or alanine conferred YFV with resistance to BDAA without apparent loss of replication fitness in cultured mammalian cells. However, replacement of P219 with glycine conferred BDAA resistance with significant loss of replication ability. Bioinformatics analysis predicts that the P219 amino acid is localized at the endoplasmic reticulum lumen side of the fifth putative transmembrane domain of NS4B, and the mutation may render the viral protein incapable of interacting with BDAA. Our studies thus revealed an important role and the structural basis for the NS4B protein in supporting YFV replication. Moreover, in YFV-infected hamsters, oral administration of BDAA protected 90% of the animals from death, significantly reduced viral load by greater than 2 logs, and attenuated virus infection-induced liver injury and body weight loss. The encouraging preclinical results thus warrant further development of BDAA or its derivatives as antiviral agents to treat yellow fever. IMPORTANCE Yellow fever is an acute viral hemorrhagic disease which threatens approximately 1 billion people living in tropical areas of Africa and Latin America. Although a highly effective yellow fever vaccine has been available for more than 7 decades, the low vaccination rate fails to prevent outbreaks in at-risk regions. It has been estimated that up to 1.7 million YFV infections occur in Africa each year, resulting in 29,000 to 60,000 deaths. Thus far, there is no specific antiviral treatment for yellow fever. To cope with this medical challenge, we identified a benzodiazepine compound that selectively inhibits YFV by targeting the viral NS4B protein. To our knowledge, this is the first report demonstrating in vivo safety and antiviral efficacy of a YFV NS4B inhibitor in an animal model. We have thus reached a critical milestone toward the development of specific antiviral therapeutics for clinical management of yellow fever.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Two mutations in NS2B are responsible for attenuation of the yellow fever virus (YFV) vaccine strain 17D.PLoS Pathog. 2025 Jul 31;21(7):e1013373. doi: 10.1371/journal.ppat.1013373. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40743291 Free PMC article.
-
Mechanism of Inhibition of the Active Triphosphate Form of 2'-α-Fluoro,2'-β-bromouridine against Yellow Fever Virus RNA-Dependent RNA Polymerase.ACS Infect Dis. 2025 Jun 13;11(6):1528-1538. doi: 10.1021/acsinfecdis.5c00086. Epub 2025 May 5. ACS Infect Dis. 2025. PMID: 40323779 Free PMC article.
-
Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages.Elife. 2025 Jun 19;13:RP103714. doi: 10.7554/eLife.103714. Elife. 2025. PMID: 40536105 Free PMC article.
-
Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses.Cochrane Database Syst Rev. 2023 Sep 12;9(9):CD015226. doi: 10.1002/14651858.CD015226.pub2. Cochrane Database Syst Rev. 2023. PMID: 37696529 Free PMC article.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
Cited by
-
Pan-serotype dengue virus inhibitor JNJ-A07 targets NS4A-2K-NS4B interaction with NS2B/NS3 and blocks replication organelle formation.Nat Commun. 2024 Jul 19;15(1):6080. doi: 10.1038/s41467-024-50437-3. Nat Commun. 2024. PMID: 39030239 Free PMC article.
-
Enhancing the antiviral potency of ER α-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo.Antiviral Res. 2018 Feb;150:112-122. doi: 10.1016/j.antiviral.2017.12.008. Epub 2017 Dec 15. Antiviral Res. 2018. PMID: 29253498 Free PMC article.
-
Flavivirus NS4B protein: Structure, function, and antiviral discovery.Antiviral Res. 2022 Nov;207:105423. doi: 10.1016/j.antiviral.2022.105423. Epub 2022 Sep 27. Antiviral Res. 2022. PMID: 36179934 Free PMC article. Review.
-
What Does the Future Hold for Yellow Fever Virus? (II).Genes (Basel). 2018 Aug 21;9(9):425. doi: 10.3390/genes9090425. Genes (Basel). 2018. PMID: 30134625 Free PMC article. Review.
-
A yellow fever virus NS4B inhibitor not only suppresses viral replication, but also enhances the virus activation of RIG-I-like receptor-mediated innate immune response.PLoS Pathog. 2022 Jan 21;18(1):e1010271. doi: 10.1371/journal.ppat.1010271. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35061864 Free PMC article.
References
-
- Reinhardt B, Jaspert R, Niedrig M, Kostner C, L'Age-Stehr J. 1998. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 56:159–167. doi: 10.1002/(SICI)1096-9071(199810)56:2<159::AID-JMV10>3.0.CO;2-B. - DOI - PubMed
-
- Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, Staples JE, Tomori O, Wilder-Smith A, Monath TP, Informal WHO Working Group on Geographic Risk for Yellow Fever . 2011. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis 11:622–632. doi: 10.1016/S1473-3099(11)70147-5. - DOI - PubMed
-
- Maciel M Jr, Cruz Fda S, Cordeiro MT, da Motta MA, Cassemiro KM, Maia Rde C, de Figueiredo RC, Galler R, Freire Mda S, August JT, Marques ET, Dhalia R. 2015. A DNA vaccine against yellow fever virus: development and evaluation. PLoS Negl Trop Dis 9:e0003693. doi: 10.1371/journal.pntd.0003693. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

