Postnatal Chick Choroids Exhibit Increased Retinaldehyde Dehydrogenase Activity During Recovery From Form Deprivation Induced Myopia
- PMID: 27654415
- PMCID: PMC5032914
- DOI: 10.1167/iovs.16-19395
Postnatal Chick Choroids Exhibit Increased Retinaldehyde Dehydrogenase Activity During Recovery From Form Deprivation Induced Myopia
Abstract
Purpose: Increases in retinaldehyde dehydrogenase 2 (RALDH2) transcript in the chick choroid suggest that RALDH2 may be responsible for increases observed in all-trans-retinoic acid (atRA) synthesis during recovery from myopic defocus. The purpose of the present study was to examine RALDH2 protein expression, RALDH activity, and distribution of RALDH2 cells in control and recovering chick ocular tissues.
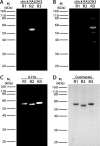
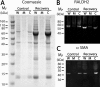
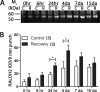
Methods: Myopia was induced in White Leghorn chicks for 10 days, followed by up to 15 days of unrestricted vision (recovery). Expression of RALDH isoforms in chick ocular tissues was evaluated by Western blot. Catalytic activity of RALDH was measured in choroidal cytosol fractions using an in vitro atRA synthesis assay together with HPLC quantification of synthesized atRA. Distribution of RALDH2 cells throughout the choroid was evaluated by immunohistochemistry.
Results: RALDH2 was expressed predominately in the chick choroid (P < 0.001) and increased after 24 hours and 4 days of recovery (76%, 74%, and 165%, respectively; P < 0.05). Activity of RALDH was detected solely in the choroid and was elevated at 3 and 7 days of recovery compared to controls (70% and 48%, respectively; P < 0.05). The number of RALDH2 immunopositive cells in recovering choroids was increased at 24 hours and 4 to 15 days of recovery (P < 0.05) and were concentrated toward the RPE side compared to controls.
Conclusions: The results of this study suggest that RALDH2 is the major RALDH isoform in the chick choroid and is responsible for the increased RALDH activity seen during recovery.
Figures





References
-
- O'Leary DJ,, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979; 35: 1478–1479. - PubMed
-
- Rabin J,, Van Sluyters RC,, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981; 20: 561–564. - PubMed
-
- Rasooly R,, BenEzra D. Congenital and traumatic cataract. The effect on ocular axial length. Arch Ophthalmol. 1988; 106: 1066–1068. - PubMed
-
- Twomey JM,, Gilvarry A,, Restori M,, Kirkness CM,, Moore AT,, Holden AL. Ocular enlargement following infantile corneal opacification. Eye (Lond). 1990; 4: 497–503. - PubMed
-
- Norton TT,, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995; 35: 1271–1281. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

