Lack of R-Ras Leads to Increased Vascular Permeability in Ischemic Retinopathy
- PMID: 27654416
- PMCID: PMC5032915
- DOI: 10.1167/iovs.16-19212
Lack of R-Ras Leads to Increased Vascular Permeability in Ischemic Retinopathy
Abstract
Purpose: The role of R-Ras in retinal angiogenesis and vascular permeability was evaluated in an oxygen-induced retinopathy (OIR) model using R-Ras knockout (KO) mice and in human diabetic neovascular membranes.
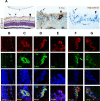
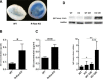
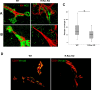
Methods: Mice deficient for R-Ras and their wild-type (WT) littermates were subjected to 75% oxygen from postnatal day 7 (P7) to P12 and then returned to room air. At P17 retinal vascularization was examined from whole mounts, and retinal vascular permeability was studied using Miles assay. Real-time RT-PCR, Western blotting, and immunohistochemistry were used to assess the expression of R-Ras in retina during development or in the OIR model. The degree of pericyte coverage and vascular endothelial (VE)-cadherin expression on WT and R-Ras KO retinal blood vessels was quantified using confocal microscopy. The correlation of R-Ras with vascular endothelial growth factor receptor 2 (VEGFR2) and human serum albumin on human proliferative diabetic retinopathy membranes was assessed using immunohistochemistry.
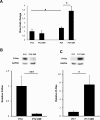
Results: In retina, R-Ras expression was mostly restricted to the vasculature. Retinal vessels in the R-Ras KO mice were significantly more permeable than WT controls in the OIR model. A significant reduction in the direct physical contact between pericytes and blood vessel endothelium as well as reduced VE-cadherin immunostaining was found in R-Ras-deficient mice. In human proliferative diabetic retinopathy neovascular membranes, R-Ras expression negatively correlated with increased vascular leakage and expression of VEGFR2, a marker of blood vessel immaturity.
Conclusions: Our results suggest that R-Ras has a role in controlling retinal vessel maturation and stabilization in ischemic retinopathy and provides a potential target for pharmacologic manipulation to treat diabetic retinopathy.
Figures


Similar articles
-
Proteomics Analysis of R-Ras Deficiency in Oxygen Induced Retinopathy.Int J Mol Sci. 2023 Apr 26;24(9):7914. doi: 10.3390/ijms24097914. Int J Mol Sci. 2023. PMID: 37175621 Free PMC article.
-
Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6625-32. doi: 10.1167/iovs.09-4900. Epub 2010 Jul 7. Invest Ophthalmol Vis Sci. 2010. PMID: 20610844 Free PMC article.
-
Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin.Elife. 2020 Apr 21;9:e54056. doi: 10.7554/eLife.54056. Elife. 2020. PMID: 32312382 Free PMC article.
-
[Pathological role of apelin in angiogenic eye disease].Yakugaku Zasshi. 2011;131(8):1201-6. doi: 10.1248/yakushi.131.1201. Yakugaku Zasshi. 2011. PMID: 21804324 Review. Japanese.
-
Exploration of Oxygen-Induced Retinopathy Model to Discover New Therapeutic Drug Targets in Retinopathies.Front Pharmacol. 2020 Jun 11;11:873. doi: 10.3389/fphar.2020.00873. eCollection 2020. Front Pharmacol. 2020. PMID: 32595503 Free PMC article. Review.
Cited by
-
Lung endothelial cells regulate pulmonary fibrosis through FOXF1/R-Ras signaling.Nat Commun. 2023 May 4;14(1):2560. doi: 10.1038/s41467-023-38177-2. Nat Commun. 2023. PMID: 37137915 Free PMC article.
-
Spatial transcriptomics reveals regionally altered gene expression that drives retinal degeneration.Commun Biol. 2025 Apr 18;8(1):629. doi: 10.1038/s42003-025-07887-2. Commun Biol. 2025. PMID: 40251274 Free PMC article.
-
Prolonged activation of cAMP signaling leads to endothelial barrier disruption via transcriptional repression of RRAS.FASEB J. 2018 May 18;32(11):fj201700818RRR. doi: 10.1096/fj.201700818RRR. Online ahead of print. FASEB J. 2018. PMID: 29775418 Free PMC article.
-
Animal Models of Retinopathy of Prematurity: Advances and Metabolic Regulators.Biomedicines. 2024 Aug 23;12(9):1937. doi: 10.3390/biomedicines12091937. Biomedicines. 2024. PMID: 39335451 Free PMC article. Review.
-
Akt3 activation by R-Ras in an endothelial cell enforces quiescence and barrier stability of neighboring endothelial cells via Jagged1.Cell Rep. 2024 Mar 26;43(3):113837. doi: 10.1016/j.celrep.2024.113837. Epub 2024 Feb 24. Cell Rep. 2024. PMID: 38402584 Free PMC article.
References
-
- Ferrara N,, Davis–Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18: 4–25. - PubMed
-
- Keck PJ,, Hauser SD,, Krivi G,, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989; 246: 1309–1312. - PubMed
-
- Vinores SA,, Chan CC,, Vinores MA,, et al. Increased vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGFbeta) in experimental autoimmune uveoretinitis: upregulation of VEGF without neovascularization. J Neuroimmunol. 1998; 89: 43–50. - PubMed
-
- Arevalo JF,, Maia M,, Flynn HW, Jr,, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008; 92: 213–216. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

