Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway
- PMID: 27654511
- PMCID: PMC5031967
- DOI: 10.1038/srep33823
Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway
Abstract
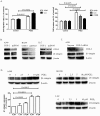
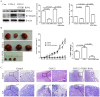
Cyclooxygenase-2 (COX-2) has been implicated in cell invasion in non-small-cell lung cancer (NSCLC). However, the mechanism is unclear. The present study investigated the effect of COX-2 on β1-integrin expression and cell invasion in NSCLC. COX-2 and β1-integrin were co-expressed in NSCLC tissues. COX-2 overexpression or Prostaglandin E2 (PGE2) treatment increased β1-integrin expression in NSCLC cell lines. β1-integrin silencing suppressed COX-2-mediated tumour growth and cancer cell invasion in vivo and in vitro. Prostaglandin E Receptor EP1 transfection or treatment with EP1 agonist mimicked the effect of PGE2 treatment. EP1 siRNA blocked PGE2-mediated β1-integrin expression. EP1 agonist treatment promoted Erk1/2, p38 phosphorylation and E2F-1 expression. MEK1/2 and p38 inhibitors suppressed EP1-mediated β1-integrin expression. E2F-1 silencing suppressed EP1-mediated FoxC2 and β1-integrin upregulation. ChIP and Luciferase Reporter assays identified that EP1 agonist treatment induced E2F-1 binding to FoxC2 promotor directly and improved FoxC2 transcription. FoxC2 siRNA suppressed β1-integrin expression and EP1-mediated cell invasion. Immunohistochemistry showed E2F-1, FoxC2, and EP1R were all highly expressed in the NSCLC cases. This study suggested that COX-2 upregulates β1-integrin expression and cell invasion in NSCLC by activating the MAPK/E2F-1 signalling pathway. Targeting the COX-2/EP1/PKC/MAPK/E2F-1/FoxC2/β1-integrin pathway might represent a new therapeutic strategy for the prevention and treatment of this cancer.
Figures




Similar articles
-
Prostaglandin E2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway.Sci Rep. 2014 Oct 7;4:6538. doi: 10.1038/srep06538. Sci Rep. 2014. PMID: 25289898 Free PMC article.
-
Prostaglandin E2 upregulates β1 integrin expression via the E prostanoid 1 receptor/nuclear factor κ-light-chain-enhancer of activated B cells pathway in non-small-cell lung cancer cells.Mol Med Rep. 2014 May;9(5):1729-36. doi: 10.3892/mmr.2014.2000. Epub 2014 Feb 28. Mol Med Rep. 2014. PMID: 24584670
-
Inhibition of PKC/MEK pathway suppresses β1-integrin and mitigates breast cancer cells proliferation.Toxicol Rep. 2021 Jul 21;8:1530-1537. doi: 10.1016/j.toxrep.2021.07.012. eCollection 2021. Toxicol Rep. 2021. PMID: 34408972 Free PMC article.
-
Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer.Biochim Biophys Acta. 2006 Aug;1766(1):104-19. doi: 10.1016/j.bbcan.2006.05.002. Epub 2006 May 23. Biochim Biophys Acta. 2006. PMID: 16859832 Review.
-
COX-2 in lung cancer: Mechanisms, development, and targeted therapies.Chronic Dis Transl Med. 2024 Mar 12;10(4):281-292. doi: 10.1002/cdt3.120. eCollection 2024 Dec. Chronic Dis Transl Med. 2024. PMID: 39429482 Free PMC article. Review.
Cited by
-
Cyclooxygenase-2 expression is associated with chemoresistance through cancer stemness property in hypopharyngeal carcinoma.Oncol Lett. 2021 Jul;22(1):533. doi: 10.3892/ol.2021.12794. Epub 2021 May 17. Oncol Lett. 2021. PMID: 34084214 Free PMC article.
-
EP1 receptor is involved in prostaglandin E2-induced osteosarcoma growth.Bosn J Basic Med Sci. 2019 Aug 20;19(3):265-273. doi: 10.17305/bjbms.2019.4177. Bosn J Basic Med Sci. 2019. PMID: 30995899 Free PMC article.
-
Genome-Wide Screen of miRNAs and Targeting mRNAs Reveals the Negatively Regulatory Effect of miR-130b-3p on PTEN by PI3K and Integrin β1 Signaling Pathways in Bladder Carcinoma.Int J Mol Sci. 2016 Dec 31;18(1):78. doi: 10.3390/ijms18010078. Int J Mol Sci. 2016. PMID: 28042869 Free PMC article.
-
Celecoxib prevents malignant progression of smoking-induced lung tumors via suppression of the COX-2/PGE2 signaling pathway in mice.Front Immunol. 2025 Mar 19;16:1557790. doi: 10.3389/fimmu.2025.1557790. eCollection 2025. Front Immunol. 2025. PMID: 40176805 Free PMC article.
-
Interplay between cyclooxygenase‑2 and microRNAs in cancer (Review).Mol Med Rep. 2021 May;23(5):347. doi: 10.3892/mmr.2021.11986. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760116 Free PMC article.
References
-
- Forman D. & Ferlay J. The global and regional burden of cancer. In: Stewart B. W., Wild C. P., Editors. World Cancer Report 2014. Lyon: International agency for research on cancer 26–69 (2014).
-
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA. Cancer J. Clin. 65, 5–29 (2015). - PubMed
-
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA. Cancer J. Clin. 64, 9–29 (2014). - PubMed
-
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2013. CA. Cancer J. Clin. 63, 11–30 (2013). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

