Ex vivo study of human visceral nociceptors
- PMID: 27654583
- PMCID: PMC5754853
- DOI: 10.1136/gutjnl-2016-311629
Ex vivo study of human visceral nociceptors
Abstract
Objective: The development of effective visceral analgesics free of deleterious gut-specific side effects is a priority. We aimed to develop a reproducible methodology to study visceral nociception in human tissue that could aid future target identification and drug evaluation.
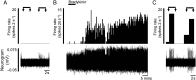
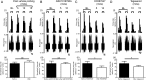
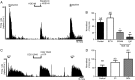
Design: Electrophysiological (single unit) responses of visceral afferents to mechanical (von Frey hair (VFH) and stretch) and chemical (bradykinin and ATP) stimuli were examined. Thus, serosal afferents (putative nociceptors) were used to investigate the effect of tegaserod, and transient receptor potential channel, vanilloid 4 (TRPV4) modulation on mechanical responses.
Results: Two distinct afferent fibre populations, serosal (n=23) and muscular (n=21), were distinguished based on their differences in sensitivity to VFH probing and tissue stretch. Serosal units displayed sensitivity to key algesic mediators, bradykinin (6/14 units tested) and ATP (4/10), consistent with a role as polymodal nociceptors, while muscular afferents are largely insensitive to bradykinin (0/11) and ATP (1/10). Serosal nociceptor mechanosensitivity was attenuated by tegaserod (-20.8±6.9%, n=6, p<0.05), a treatment for IBS, or application of HC067047 (-34.9±10.0%, n=7, p<0.05), a TRPV4 antagonist, highlighting the utility of the preparation to examine the mechanistic action of existing drugs or novel analgesics. Repeated application of bradykinin or ATP produced consistent afferent responses following desensitisation to the first application, demonstrating their utility as test stimuli to evaluate analgesic activity.
Conclusions: Functionally distinct subpopulations of human visceral afferents can be demonstrated and could provide a platform technology to further study nociception in human tissue.
Keywords: ABDOMINAL PAIN; ELECTROPHYSIOLOGY; NEUROGASTROENTEROLOGY; VISCERAL NOCICEPTION; VISCERAL SENSITIVITY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
