Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration
- PMID: 27654856
- PMCID: PMC5031982
- DOI: 10.1038/srep33830
Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration
Erratum in
-
Corrigendum: Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration.Sci Rep. 2017 Jan 19;7:40460. doi: 10.1038/srep40460. Sci Rep. 2017. PMID: 28102236 Free PMC article. No abstract available.
Abstract
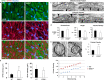
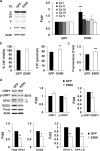
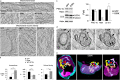
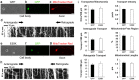
Mutations in optineurin (OPTN) are linked to the pathology of primary open angle glaucoma (POAG) and amyotrophic lateral sclerosis. Emerging evidence indicates that OPTN mutation is involved in accumulation of damaged mitochondria and defective mitophagy. Nevertheless, the role played by an OPTN E50K mutation in the pathogenic mitochondrial mechanism that underlies retinal ganglion cell (RGC) degeneration in POAG remains unknown. We show here that E50K expression induces mitochondrial fission-mediated mitochondrial degradation and mitophagy in the axons of the glial lamina of aged E50K-tg mice in vivo. While E50K activates the Bax pathway and oxidative stress, and triggers dynamics alteration-mediated mitochondrial degradation and mitophagy in RGC somas in vitro, it does not affect transport dynamics and fission of mitochondria in RGC axons in vitro. These results strongly suggest that E50K is associated with mitochondrial dysfunction in RGC degeneration in synergy with environmental factors such as aging and/or oxidative stress.
Figures




Similar articles
-
Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line.Biochem Biophys Res Commun. 2018 Sep 18;503(4):2690-2697. doi: 10.1016/j.bbrc.2018.08.025. Epub 2018 Aug 9. Biochem Biophys Res Commun. 2018. PMID: 30100066 Free PMC article.
-
The E50K optineurin mutation impacts autophagy-mediated degradation of TDP-43 and leads to RGC apoptosis in vivo and in vitro.Cell Death Discov. 2021 Mar 15;7(1):49. doi: 10.1038/s41420-021-00432-0. Cell Death Discov. 2021. PMID: 33723228 Free PMC article.
-
Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma.Hum Mol Genet. 2013 Sep 1;22(17):3559-67. doi: 10.1093/hmg/ddt210. Epub 2013 May 12. Hum Mol Genet. 2013. PMID: 23669351
-
Altered Functions and Interactions of Glaucoma-Associated Mutants of Optineurin.Front Immunol. 2018 Jun 6;9:1287. doi: 10.3389/fimmu.2018.01287. eCollection 2018. Front Immunol. 2018. PMID: 29951055 Free PMC article. Review.
-
Defects in autophagy caused by glaucoma-associated mutations in optineurin.Exp Eye Res. 2016 Mar;144:54-63. doi: 10.1016/j.exer.2015.08.020. Epub 2015 Aug 21. Exp Eye Res. 2016. PMID: 26302410 Review.
Cited by
-
Dysfunction of Optineurin in Amyotrophic Lateral Sclerosis and Glaucoma.Front Immunol. 2018 May 23;9:1017. doi: 10.3389/fimmu.2018.01017. eCollection 2018. Front Immunol. 2018. PMID: 29875767 Free PMC article. Review.
-
The Role of MicroRNAs in Mitochondria-Mediated Eye Diseases.Front Cell Dev Biol. 2021 Jun 18;9:653522. doi: 10.3389/fcell.2021.653522. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34222230 Free PMC article. Review.
-
Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson's disease.NPJ Parkinsons Dis. 2018 Mar 26;4:9. doi: 10.1038/s41531-018-0044-6. eCollection 2018. NPJ Parkinsons Dis. 2018. PMID: 29872690 Free PMC article.
-
Autophagy and Aging: Roles in Skeletal Muscle, Eye, Brain and Hepatic Tissue.Front Cell Dev Biol. 2021 Oct 28;9:752962. doi: 10.3389/fcell.2021.752962. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778264 Free PMC article. Review.
-
Optineurin: A Coordinator of Membrane-Associated Cargo Trafficking and Autophagy.Front Immunol. 2018 May 15;9:1024. doi: 10.3389/fimmu.2018.01024. eCollection 2018. Front Immunol. 2018. PMID: 29867991 Free PMC article. Review.
References
-
- Weinreb R. N. & Khaw P. T. Primary open-angle glaucoma. Lancet 363, 1711–1720 (2004). - PubMed
-
- Zhang K., Zhang L. & Weinreb R. N. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov 11, 541–559 (2012). - PubMed
-
- Maruyama H. et al.. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226 (2010). - PubMed
-
- Rezaie T. et al.. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295, 1077–1079 (2002). - PubMed
-
- Kroeber M., Ohlmann A., Russell P. & Tamm E. R. Transgenic studies on the role of optineurin in the mouse eye. Experimental Eye Research 82, 1075–1085 (2006). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

