Dscam1 Forms a Complex with Robo1 and the N-Terminal Fragment of Slit to Promote the Growth of Longitudinal Axons
- PMID: 27654876
- PMCID: PMC5031454
- DOI: 10.1371/journal.pbio.1002560
Dscam1 Forms a Complex with Robo1 and the N-Terminal Fragment of Slit to Promote the Growth of Longitudinal Axons
Abstract
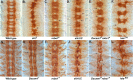
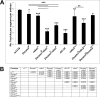
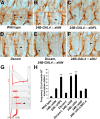
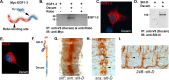
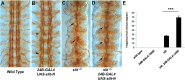
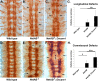
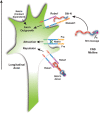
The Slit protein is a major midline repellent for central nervous system (CNS) axons. In vivo, Slit is proteolytically cleaved into N- and C-terminal fragments, but the biological significance of this is unknown. Analysis in the Drosophila ventral nerve cord of a slit allele (slit-UC) that cannot be cleaved revealed that midline repulsion is still present but longitudinal axon guidance is disrupted, particularly across segment boundaries. Double mutants for the Slit receptors Dscam1 and robo1 strongly resemble the slit-UC phenotype, suggesting they cooperate in longitudinal axon guidance, and through biochemical approaches, we found that Dscam1 and Robo1 form a complex dependent on Slit-N. In contrast, Robo1 binding alone shows a preference for full-length Slit, whereas Dscam1 only binds Slit-N. Using a variety of transgenes, we demonstrated that Dscam1 appears to modify the output of Robo/Slit complexes so that signaling is no longer repulsive. Our data suggest that the complex is promoting longitudinal axon growth across the segment boundary. The ability of Dscam1 to modify the output of other receptors in a ligand-dependent fashion may be a general principle for Dscam proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, et al. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nature neuroscience. 2005;8(3):297–304. - PubMed
-
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science (New York, NY). 2003;302(5652):1984–8. - PubMed
-
- Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Developmental biology. 2008;313(1):371–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

