Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia
- PMID: 27654879
- PMCID: PMC5157093
- DOI: 10.1113/JP273164
Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia
Abstract
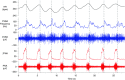
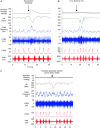
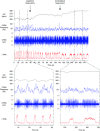
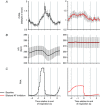
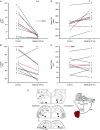
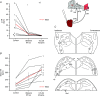
Key points: Cardiac vagal tone is a strong predictor of health, although its central origins are unknown. Respiratory-linked fluctuations in cardiac vagal tone give rise to respiratory sinus arryhthmia (RSA), with maximum tone in the post-inspiratory phase of respiration. In the present study, we investigated whether respiratory modulation of cardiac vagal tone is intrinsically linked to post-inspiratory respiratory control using the unanaesthetized working heart-brainstem preparation of the rat. Abolition of post-inspiration, achieved by inhibition of the pontine Kolliker-Fuse nucleus, removed post-inspiratory peaks in efferent cardiac vagal activity and suppressed RSA, whereas substantial cardiac vagal tone persisted. After transection of the caudal pons, part of the remaining tone was removed by inhibition of nucleus of the solitary tract. We conclude that cardiac vagal tone depends upon at least 3 sites of the pontomedullary brainstem and that a significant proportion arises independently of RSA.
Abstract: Cardiac vagal tone is a strong predictor of health, although its central origins are unknown. The rat working heart-brainstem preparation shows strong cardiac vagal tone and pronounced respiratory sinus arrhythmia. In this preparation, recordings from the cut left cardiac vagal branch showed efferent activity that peaked in post-inspiration, ∼0.5 s before the cyclic minimum in heart rate (HR). We hypothesized that respiratory modulation of cardiac vagal tone and HR is intrinsically linked to the generation of post-inspiration. Neurons in the pontine Kölliker-Fuse nucleus (KF) were inhibited with bilateral microinjections of isoguvacine (50-70 nl, 10 mm) to remove the post-inspiratory phase of respiration. This also abolished the post-inspiratory peak of cardiac vagal discharge (and cyclical HR modulation), although a substantial level of activity remained. In separate preparations with intact cardiac vagal branches but sympathetically denervated by thoracic spinal pithing, cardiac chronotropic vagal tone was quantified by HR compared to its final level after systemic atropine (0.5 μm). Bilateral KF inhibition removed 88% of the cyclical fluctuation in HR but, on average, only 52% of the chronotropic vagal tone. Substantial chronotropic vagal tone also remained after transection of the brainstem through the caudal pons. Subsequent bilateral isoguvacine injections into the nucleus of the solitary tract further reduced vagal tone: remaining sources were untraced. We conclude that cardiac vagal tone depends on neurons in at least three sites of the pontomedullary brainstem, and much of it arises independently of respiratory sinus arrhythmia.
© 2016 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures


References
-
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC & Cohen RJ (1981). Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat‐to‐beat cardiovascular control. Science 213, 220–222. - PubMed
-
- Anrep GV, Pascual W & Rossler R (1936. a). respiratory variations of the heart rate. I. – The reflex mechanism of the respiratory arrhythmia. Proc R Soc Lond Ser B – Biol Sci 119, 191–217.
-
- Anrep GV, Pascual W & Rossler R (1936. b). Respiratory variations of the heart rate. II. – The central mechanism of the respiratory arrhythmia and the inter‐relations between the central and the reflex mechanisms. Proc R Soc Lond Ser B – Biol Sci 119, 218–230.
-
- Baekey DM, Dick TE & Paton JFR (2008). Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93, 803–816. - PubMed
-
- Barman SM & Gebber GL (2000). “Rapid” rhythmic discharges of sympathetic nerves: sources, mechanisms of generation, and physiological relevance. J Biol Rhythms 15, 365–379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

