Ketoprofen for episodic tension-type headache in adults
- PMID: 27654948
- PMCID: PMC6457784
- DOI: 10.1002/14651858.CD012190.pub2
Ketoprofen for episodic tension-type headache in adults
Abstract
Background: Tension-type headache (TTH) affects about 1 person in 5 worldwide. It is divided into infrequent episodic TTH (fewer than one headache day per month), frequent episodic TTH (2 to 14 headache days per month), and chronic TTH (15 headache days a month or more). Ketoprofen is one of a number of analgesics suggested for acute treatment of headaches in frequent episodic TTH.
Objectives: To assess the efficacy and safety of ketoprofen for treatment of episodic TTH in adults compared with placebo or any active comparator.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and the Oxford Pain Relief Database up to May 2016, and also reference lists of relevant published studies and reviews. We sought unpublished studies by asking personal contacts and searching online clinical trial registers and manufacturers' websites.
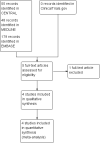
Selection criteria: We included randomised, double-blind, placebo-controlled studies (parallel-group or cross-over) using oral ketoprofen for symptomatic relief of an acute episode of TTH. Studies had to be prospective, with participants aged 18 years or over, and include at least 10 participants per treatment arm.
Data collection and analysis: Two review authors independently assessed studies for inclusion and extracted data. We used the numbers of participants achieving each outcome to calculate the risk ratio (RR) and number needed to treat for one additional beneficial outcome (NNT) or one additional harmful outcome (NNH) for oral ketoprofen compared to placebo or an active intervention for a range of outcomes, predominantly those recommended by the International Headache Society (IHS).We assessed the evidence using GRADE and created a 'Summary of findings' table.
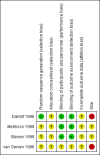
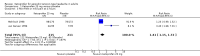
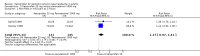
Main results: We included four studies, all of which enrolled adults with frequent episodic TTH. They all specified using the IHS diagnostic criteria and reported mean baseline pain of at least moderate intensity. While 1253 people with TTH participated in these studies, the numbers available for any analysis were lower than this because outcomes were inconsistently reported and because many participants received active comparators.None of the included studies were at low risk of bias across all domains considered, although for most studies and domains this was likely to be due to inadequate reporting rather than poor methods. We judged one study to be at high risk of bias due to small size.Useful information was available only for ketoprofen 25 mg. For the IHS preferred outcome of being pain-free at two hours the NNT for ketoprofen 25 mg compared with placebo was 9.0 (95% confidence interval (CI) 4.8 to 72) in two studies (272 participants; low quality evidence). The NNT was 3.7 (95% CI 2.6 to 6.3) for pain-free or mild pain at two hours in two studies (272 participants; moderate quality evidence). Fewer people needed rescue medication with ketoprofen 25 mg than with placebo, with a number needed to treat to prevent one event (NNTp) of 6.2 (95% CI 4.3 to 11) in three studies (605 participants; moderate quality evidence). The number of participants reporting any adverse event was higher with ketoprofen 25 mg than placebo (NNH 15, (95% CI 8.7 to 45)) in three studies (651 participants with 66 events; low quality evidence). Most events were of mild to moderate intensity.Ketoprofen 25 mg was not different from paracetamol 1000 mg in two studies with 276 participants for any efficacy outcomes (low to moderate quality evidence); the RR for pain-free at two hours was 1.3 (95% CI 0.9 to 2.0). The number of participants reporting any adverse event was higher with ketoprofen 25 mg than with paracetamol (NNH 17, 95% CI 8.9 to 130)) in two studies (582 participants, 68 events; low quality evidence).Studies reported no serious adverse events.We judged the quality of the evidence comparing ketoprofen 25 mg with placebo or paracetamol 1000 mg as moderate to very low. Where evidence was downgraded it was because of the small number of studies and events.
Authors' conclusions: Ketoprofen 25 mg provided a small benefit compared with placebo in terms of being pain-free at two hours or having mild or no pain at two hours for people with frequent episodic TTH who have an acute headache of moderate or severe intensity. Its use was associated with more people experiencing adverse events. Ketoprofen 25 mg was not superior to paracetamol 1000 mg for any efficacy outcome.
Conflict of interest statement
LV: none known.
SD: none known.
RAM has received grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Figures









Update of
- doi: 10.1002/14651858.CD012190
Similar articles
-
Aspirin for acute treatment of episodic tension-type headache in adults.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD011888. doi: 10.1002/14651858.CD011888.pub2. Cochrane Database Syst Rev. 2017. PMID: 28084009 Free PMC article.
-
Paracetamol (acetaminophen) for acute treatment of episodic tension-type headache in adults.Cochrane Database Syst Rev. 2016 Jun 16;2016(6):CD011889. doi: 10.1002/14651858.CD011889.pub2. Cochrane Database Syst Rev. 2016. PMID: 27306653 Free PMC article.
-
Ibuprofen for acute treatment of episodic tension-type headache in adults.Cochrane Database Syst Rev. 2015 Jul 31;2015(7):CD011474. doi: 10.1002/14651858.CD011474.pub2. Cochrane Database Syst Rev. 2015. PMID: 26230487 Free PMC article.
-
Single fixed-dose oral dexketoprofen plus tramadol for acute postoperative pain in adults.Cochrane Database Syst Rev. 2016 Sep 22;9(9):CD012232. doi: 10.1002/14651858.CD012232.pub2. Cochrane Database Syst Rev. 2016. PMID: 27654994 Free PMC article. Review.
-
Single-dose intravenous ketorolac for acute postoperative pain in adults.Cochrane Database Syst Rev. 2021 May 17;5(5):CD013263. doi: 10.1002/14651858.CD013263.pub2. Cochrane Database Syst Rev. 2021. PMID: 33998669 Free PMC article.
Cited by
-
Variables associated with use of symptomatic medication during a headache attack in individuals with tension-type headache: a European study.BMC Neurol. 2020 Feb 1;20(1):43. doi: 10.1186/s12883-020-1624-8. BMC Neurol. 2020. PMID: 32007103 Free PMC article.
-
Chinese Herbal Formula Xuefu Zhuyu for Tension-Type Headache with Qi-Stagnation and Blood-Stasis Pattern (CheruXTH): Study Protocol for a Randomized Controlled Trial.Evid Based Complement Alternat Med. 2020 Sep 11;2020:5653169. doi: 10.1155/2020/5653169. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32963565 Free PMC article.
-
Framing the numerical findings of Cochrane plain language summaries: two randomized controlled trials.BMC Med Res Methodol. 2020 May 6;20(1):101. doi: 10.1186/s12874-020-00990-4. BMC Med Res Methodol. 2020. PMID: 32375659 Free PMC article.
-
Aspirin for acute treatment of episodic tension-type headache in adults.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD011888. doi: 10.1002/14651858.CD011888.pub2. Cochrane Database Syst Rev. 2017. PMID: 28084009 Free PMC article.
References
References to studies included in this review
Dahlöf 1996 {published data only}
Mehlisch 1998 {published data only}
Steiner 1998 {published data only}
-
- Steiner TJ, Lange R. Ketoprofen (25 mg) in the symptomatic treatment of episodic tension‐type headache: double‐blind placebo‐controlled comparison with acetaminophen (1000 mg). Cephalalgia 1998;18(1):38‐43. [DOI: ] - PubMed
van Gerven 1996 {published data only}
-
- Gerven JM, Schoemaker RC, Jacobs LD, Reints A, Ouwersloot‐van der Meij MJ, Hoedemaker HG, et al. Self‐medication of a single headache episode with ketoprofen, ibuprofen or placebo, home‐monitored with an electronic patient diary. British Journal of Clinical Pharmacology 1996;42(4):475‐81. [DOI: 10.1111/j.1365-2125.1996.tb00011.x] - DOI - PMC - PubMed
References to studies excluded from this review
Lange 1995 {published data only}
-
- Lange R, Lentz R. Comparison ketoprofen, ibuprofen and naproxen sodium in the treatment of tension‐type headache. Drugs Under Experimental and Clinical Research 1995;21(3):89‐96. [PUBMED: 7555617] - PubMed
Additional references
Ayzenberg 2012
Barden 2009
BASH 2010
-
- MacGregor EA, Steiner TJ, Davies PTG, for the British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension‐type, cluster, and medication overuse headache. 3rd edition (1st revision), 2010. www.bash.org.uk/wp‐content/uploads/2012/07/10102‐BASH‐Guidelines‐update‐... (accessed 14 December 2015).
Bendtsen 2010
Brainin 2004
-
- Brainin M, Barnes M, Baron JC, Gilhus NE, Hughes R, Selmaj K, et al. for the Guideline Standards Subcommittee of the EFNS Scientific Committee. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces‐revised recommendations 2004. European Journal of Neurology 2004;11(9):577‐81. [DOI: 10.1111/j.1468-1331.2004.00867.x] - DOI - PubMed
Cook 1995
Cristofolini 2008
-
- Cristofolini A, Dalla Serra P, Scherillo G, Orrico D, Micciolo R. The prevalence of headache in a population of health care workers and the effects on productivity costs. La Medicina del Lavoro 2008;99(1):8‐15. - PubMed
Derry 2015
Edmeads 1993
-
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [PUBMED: 8334575] - PubMed
Elbourne 2002
Eypasch 1995
Fernández‐de‐las‐Peñas 2010
-
- Fernández‐de‐las‐Peñas C, Madeleine P, Caminero AB, Cuadrado ML, Arendt‐Nielsen L, Pareja JA. Generalized neck‐shoulder hyperalgesia in chronic tension‐type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia 2010;30(1):77‐86. [DOI: 10.1111/j.1468-2982.2009.01901.x] - DOI - PubMed
FitzGerald 2001
Forward 1998
Fumal 2008
Green 2009
-
- Green MW. Primary and secondary headaches. In: Rowland LP, Pedley TA editor(s). Merritt's Neurology. 12th Edition. Philadelphia: Lippincott Williams & Wilkins, 2009:951‐60. [ISBN: 9780781791861]
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Haag 2011
-
- Haag G, Diener HC, May A, Meyer C, Morck H, Straube A, et al. Self‐medication of migraine and tension‐type headache: summary of the evidence‐based recommendations of the Deutsche Migräne und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft für Neurologie (DGN), the Österreichische Kopfschmerzgesellschaft (ÖKSG) and the Schweizerische Kopfwehgesellschaft (SKG). Journal of Headache and Pain 2011;12(2):201‐17. [DOI: 10.1007/s10194-010-0266-4] - DOI - PMC - PubMed
Harpole 2005
Hawkey 1999
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IHS 2004
IHS 2010
-
- Bendtsen L, Bigal ME, Cerbo R, Diener HC, Holroyd K, Lampl C, et al. on behalf of the International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in tension‐type headache: second edition. Cephalalgia 2010;30(1):1‐16. [DOI: 10.1111/j.1468-2982.2009.01948.x] - DOI - PubMed
IHS 2013
Jadad 1996a
Jadad 1996b
Kernick 2008
L'Abbé 1987
Lavados 1998
Lyngberg 2005
Mannix 2001
Mbewe 2015
Monteith 2010
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2011
Moore 2012
Moore 2013
Moore 2014
Morris 1995
-
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal Books, 1995:50‐63. [ISBN: 0‐7279‐0222‐0] - PMC - PubMed
Oshinaike 2014
PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 1 May 2016).
Pop 2002
-
- Pop PH, Gierveld CM, Karis HA, Tiedink HG. Epidemiological aspects of headache in a workplace setting and the impact on the economic loss. European Journal of Neurology 2002;9(2):171‐4. - PubMed
Rao 2015
Rasmussen 2001
-
- Rasmussen BK. Epidemiology of headache. Cephalalgia 2001;21(7):774‐7. [PUBMED: 11595011] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2005
Sahler 2012
Sarzi‐Puttini 2013
-
- Sarzi‐Puttini P, Atzeni F, Lanata L, Bagnasco M. Efficacy of ketoprofen vs. ibuprofen and diclofenac: a systematic review of the literature and meta‐analysis. Clinical and Experimental Pharmacology 2013;31(5):731‐8. [PUBMED: 23711416] - PubMed
Schmidt‐Wilcke 2005
Schwartz 1998
Steiner 2004
Steiner 2011
Stephens 2016
Stovner 2007
Tramèr 1997
Verhagen 2006
-
- Verhagen AP, Damen L, Berger MY, Passchier J, Merlijn V, Koes BW. Is any one analgesic superior for episodic tension‐type headache?. Journal of Family Practice 2006;55(12):1064‐72. [PUBMED: 17137543] - PubMed
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
WHO 2015
-
- World Health Organization. WHO model list of essential medicines. 19th edition. April 2015. www.who.int/medicines/publications/essentialmedicines/en/index.html (accessed 14 December 2015).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

