Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases
- PMID: 27654968
- PMCID: PMC5287290
- DOI: 10.1001/jamaneurol.2016.3338
Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases
Abstract
Importance: The causes of cognitive impairment in dementia with Lewy bodies (DLB) and Parkinson disease (PD) are multifactorial. Tau pathologic changes are commonly observed at autopsy in individuals with DLB and PD dementia, but their contribution to these diseases during life is unknown.
Objective: To contrast tau aggregation in DLB, cognitively impaired persons with PD (PD-impaired), cognitively normal individuals with PD (PD-normal), and healthy persons serving as control participants, and to evaluate the association between tau aggregation, amyloid deposition, and cognitive function.
Design, setting, and participants: This cross-sectional study was conducted from January 1, 2014, to April 28, 2016, in a tertiary care center's memory and movement disorders units. Twenty-four patients with Lewy body disease (7 DLB, 8 PD-impaired, and 9 PD-normal) underwent multimodal brain imaging, cognitive testing, and neurologic evaluation, and imaging measures were compared with those of an independently acquired group of 29 controls with minimal brain amyloid burden as measured with carbon 11-labeled Pittsburgh Compound B ([11C]PiB) positron emission tomography (PET).
Exposures: Imaging with fluorine 18-labeled AV-1451 ([18F]AV-1451) (formerly known as [18F]T807), [11C]PiB PET, magnetic resonance imaging (MRI), neurologic examination, and detailed cognitive testing using the Mini-Mental State Examination (MMSE) and Clinical Dementia Rating scale.
Main outcomes and measures: Main outcomes were differentiation of diagnostic groups on the basis of [18F]AV-1451 binding, the association of [18F]AV-1451 binding with [11C]PiB binding, and the association of [18F]AV-1451 binding with cognitive impairment. All but 3 individuals underwent amyloid imaging with [11C]PiB PET. The hypotheses being tested were formulated before data collection. Mini-Mental State Examination (range, 0-30, with 30 being best) and Clinical Dementia Rating scale sum-of-boxes scale (range, 0-18, with 0 being best) were used for assessment of cognitive function.
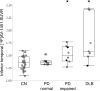
Results: In patients with DLB, cortical [18F]AV-1451 uptake was highly variable and greater than in the controls, particularly in the inferior temporal gyrus and precuneus. Foci of increased [18F]AV-1451 binding in the inferior temporal gyrus and precuneus were also evident in PD-impaired patients. Elevated cortical [18F]AV-1451 binding was observed in 4 of 17 patients with Lewy body disease with low cortical [11C]PiB retention. For DLB and PD-impaired patients, greater [18F]AV-1451 uptake in the inferior temporal gyrus and precuneus was associated with increased cognitive impairment as measured with the MMSE and the Clinical Dementia Rating scale sum-of-boxes score.
Conclusions and relevance: Patients with Lewy body disease manifest a spectrum of tau pathology. Cortical aggregates of tau are common in patients with DLB and in PD-impaired patients, even in those without elevated amyloid levels. When present, tau deposition is associated with cognitive impairment. These findings support a role for tau copathology in the Lewy body diseases.
Conflict of interest statement
BCD has provided consulting services for Merck DSMB, Forum, Ionis, Piramal, and receives royalties from Oxford University Press. RAS has provided providing consulting services for Roche, Genentech, Biogen and Bracket, Abbvie, received support from a joint NIH-Lilly-sponsored clinical trial (A4 Study – U19AG10483), and research funding from the National Institutes of Health/National Institute on Aging (R01 AG046396, P01 AG036694, P50 AG00513421) and the Alzheimer’s Association. KJ has provided consulting services for Lilly, Novartis, Janssen, Roche, Piramal, GE Healthcare, Siemens, ISIS Pharma, AZTherapy, Abbvie, Lundbeck, and Biogen, received support from a joint NIH-Lilly-sponsored clinical trial (A4 Study – U19AG10483), and received research support from National Institutes of Health/National Institute on Aging (R01 AG046396, P01 AG036694, P50 AG00513421, U19AG10483, U01AG024904-S1), Fidelity Biosciences, the Michael J. Fox Foundation, the Marr Foundation, and the Alzheimer’s Association.
Figures


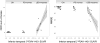
References
-
- Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102(4):355–363. - PubMed
-
- Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm (Vienna) 2002 Mar;109(3):329–39. - PubMed
-
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115(4):427–36. - PubMed
-
- Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kovari E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(10):864–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

