Interferon-γ blocks signalling through PDGFRβ in human brain pericytes
- PMID: 27654972
- PMCID: PMC5031293
- DOI: 10.1186/s12974-016-0722-4
Interferon-γ blocks signalling through PDGFRβ in human brain pericytes
Abstract
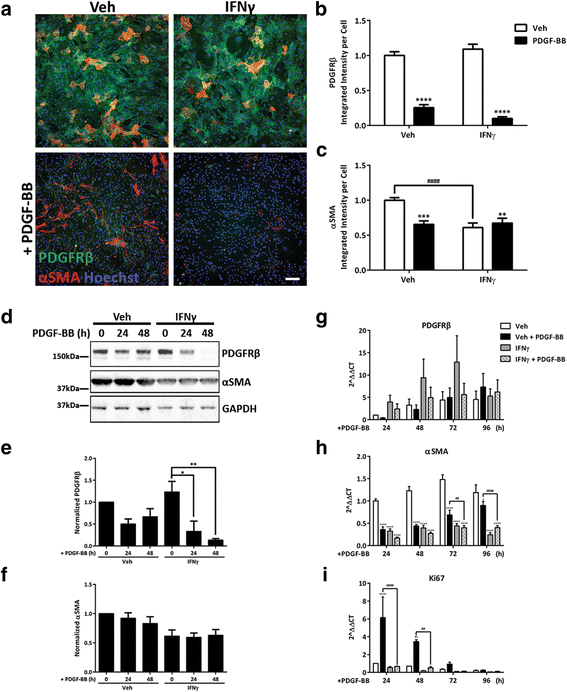
Background: Neuroinflammation and blood-brain barrier (BBB) disruption are common features of many brain disorders, including Alzheimer's disease, epilepsy, and motor neuron disease. Inflammation is thought to be a driver of BBB breakdown, but the underlying mechanisms for this are unclear. Brain pericytes are critical cells for maintaining the BBB and are immunologically active. We sought to test the hypothesis that inflammation regulates the BBB by altering pericyte biology.
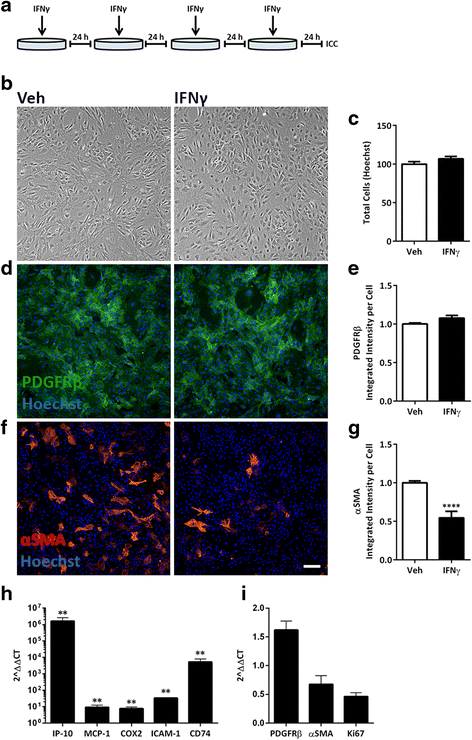
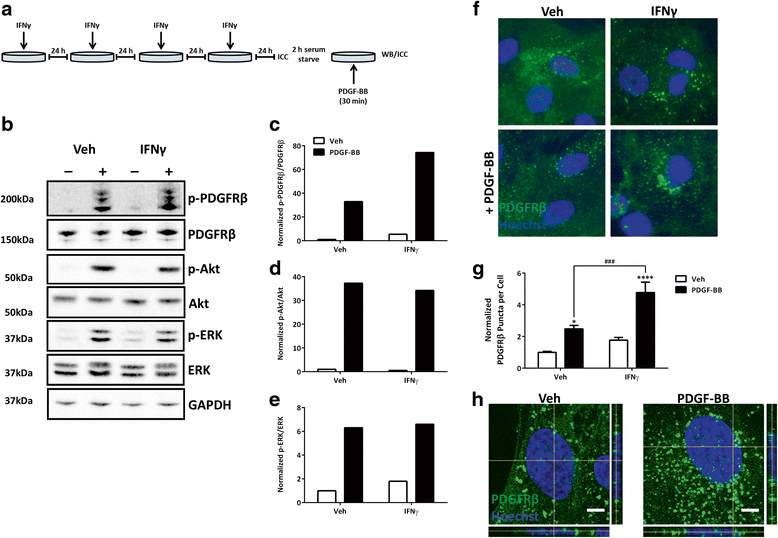
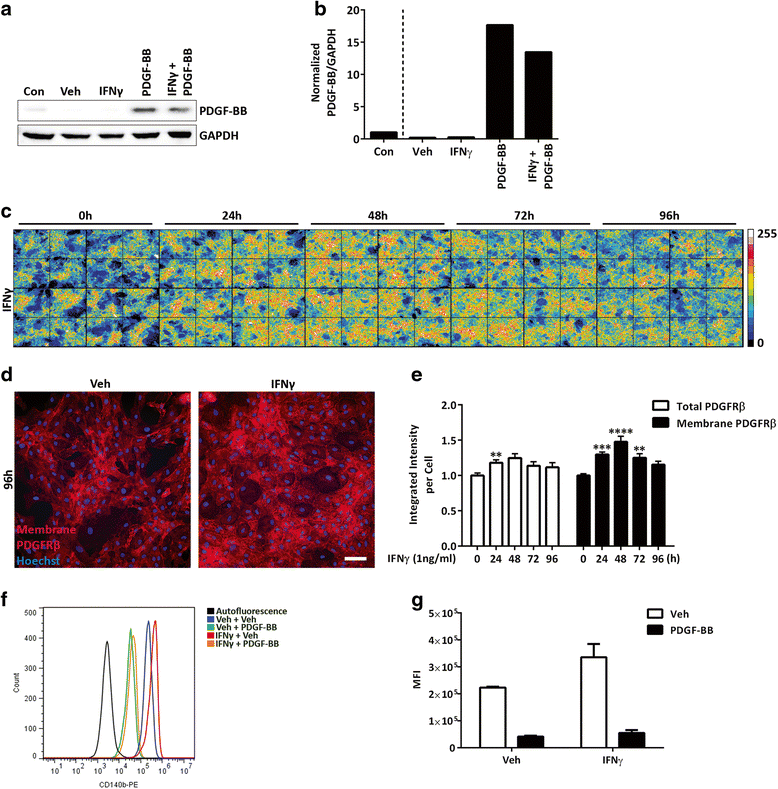
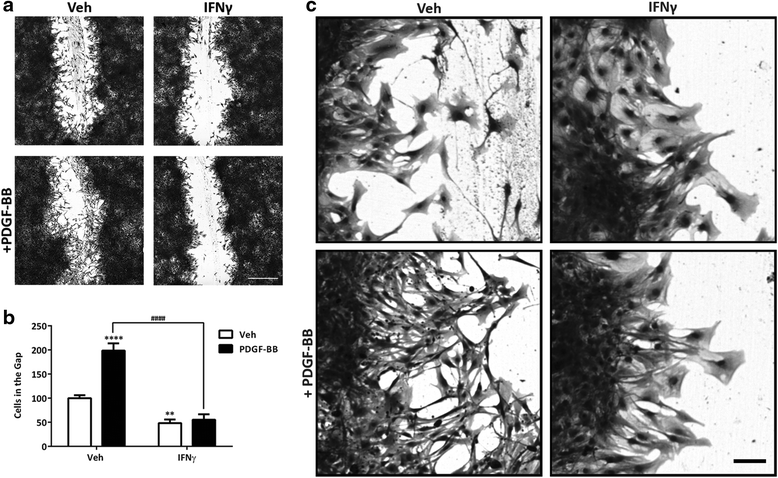
Methods: We exposed primary adult human brain pericytes to chronic interferon-gamma (IFNγ) for 4 days and measured associated functional aspects of pericyte biology. Specifically, we examined the influence of inflammation on platelet-derived growth factor receptor-beta (PDGFRβ) expression and signalling, as well as pericyte proliferation and migration by qRT-PCR, immunocytochemistry, flow cytometry, and western blotting.
Results: Chronic IFNγ treatment had marked effects on pericyte biology most notably through the PDGFRβ, by enhancing agonist (PDGF-BB)-induced receptor phosphorylation, internalization, and subsequent degradation. Functionally, chronic IFNγ prevented PDGF-BB-mediated pericyte proliferation and migration.
Conclusions: Because PDGFRβ is critical for pericyte function and its removal leads to BBB leakage, our results pinpoint a mechanism linking chronic brain inflammation to BBB dysfunction.
Keywords: Blood-brain barrier; Inflammation; Migration; Proliferation.
Figures


References
-
- Politis M, Lahiri N, Niccolini F, Su P, Wu K, Giannetti P, Scahill RI, Turkheimer FE, Tabrizi SJ, Piccini P. Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington’s disease gene carriers. Neurobiol Dis. 2015;83:115-21. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

