Neutrophil azurophilic granule exocytosis is primed by TNF-α and partially regulated by NADPH oxidase
- PMID: 27655046
- PMCID: PMC6805152
- DOI: 10.1177/1753425916668980
Neutrophil azurophilic granule exocytosis is primed by TNF-α and partially regulated by NADPH oxidase
Abstract
Neutrophil (polymorphonuclear leukocyte) activation with release of granule contents plays an important role in the pathogenesis of acute lung injury, prompting clinical trials of inhibitors of neutrophil elastase. Despite mounting evidence for neutrophil-mediated host tissue damage in a variety of disease processes, mechanisms regulating azurophilic granule exocytosis at the plasma membrane, and thus release of elastase and other proteases, are poorly characterized. We hypothesized that azurophilic granule exocytosis would be enhanced under priming conditions similar to those seen during acute inflammatory events and during chronic inflammatory disease, and selected the cytokine TNF-α to model this in vitro. Neutrophils stimulated with TNF-α alone elicited intracellular reactive oxygen species (ROS) generation and mobilization of secretory vesicles, specific, and gelatinase granules. p38 and ERK1/2 MAPK were involved in these components of priming. TNF-α priming alone did not mobilize azurophilic granules to the cell surface, but did markedly increase elastase release into the extracellular space in response to secondary stimulation with N-formyl-Met-Leu-Phe (fMLF). Priming of fMLF-stimulated elastase release was further augmented in the absence of NADPH oxidase-derived ROS. Our findings provide a mechanism for host tissue damage during neutrophil-mediated inflammation and suggest a novel anti-inflammatory role for the NADPH oxidase.
Keywords: Elastase; NADPH oxidase 2; inflammation; priming; reactive oxygen species; signaling.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
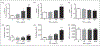


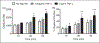


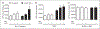
Similar articles
-
Endocytosis is required for exocytosis and priming of respiratory burst activity in human neutrophils.Inflamm Res. 2017 Oct;66(10):891-899. doi: 10.1007/s00011-017-1070-2. Epub 2017 Jun 21. Inflamm Res. 2017. PMID: 28638979
-
Frontline Science: Tumor necrosis factor-α stimulation and priming of human neutrophil granule exocytosis.J Leukoc Biol. 2017 Jul;102(1):19-29. doi: 10.1189/jlb.3HI0716-293RR. Epub 2017 Jan 17. J Leukoc Biol. 2017. PMID: 28096297 Free PMC article.
-
Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-α is partially oxygen dependent.J Innate Immun. 2011;3(3):298-314. doi: 10.1159/000321439. Epub 2010 Nov 19. J Innate Immun. 2011. PMID: 21088376 Free PMC article.
-
Review of Defective NADPH Oxidase Activity and Myeloperoxidase Release in Neutrophils From Patients With Cirrhosis.Front Immunol. 2019 May 8;10:1044. doi: 10.3389/fimmu.2019.01044. eCollection 2019. Front Immunol. 2019. PMID: 31134093 Free PMC article. Review.
-
Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane.Semin Immunopathol. 2008 Jul;30(3):279-89. doi: 10.1007/s00281-008-0118-3. Epub 2008 Jun 7. Semin Immunopathol. 2008. PMID: 18536919 Review.
Cited by
-
Therapeutic targeting of neutrophil exocytosis.J Leukoc Biol. 2020 Mar;107(3):393-408. doi: 10.1002/JLB.3RI0120-645R. Epub 2020 Jan 28. J Leukoc Biol. 2020. PMID: 31990103 Free PMC article. Review.
-
Oxidative Stress and Microvessel Barrier Dysfunction.Front Physiol. 2020 May 27;11:472. doi: 10.3389/fphys.2020.00472. eCollection 2020. Front Physiol. 2020. PMID: 32536875 Free PMC article. Review.
-
Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome.Front Immunol. 2019 Nov 22;10:2734. doi: 10.3389/fimmu.2019.02734. eCollection 2019. Front Immunol. 2019. PMID: 31824510 Free PMC article. Review.
-
Endocytosis is required for exocytosis and priming of respiratory burst activity in human neutrophils.Inflamm Res. 2017 Oct;66(10):891-899. doi: 10.1007/s00011-017-1070-2. Epub 2017 Jun 21. Inflamm Res. 2017. PMID: 28638979
-
The Impact of Hypoxia on Neutrophil Degranulation and Consequences for the Host.Int J Mol Sci. 2020 Feb 11;21(4):1183. doi: 10.3390/ijms21041183. Int J Mol Sci. 2020. PMID: 32053993 Free PMC article. Review.
References
-
- Hirche TO, Crouch EC, Espinola M, et al. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J Biol Chem 2004; 279: 27688–27698. - PubMed
-
- Kodama T, Yukioka H, Kato T, et al. Neutrophil elastase as a predicting factor for development of acute lung injury. Intern Med 2007; 46: 699–704. - PubMed
-
- Idell S, Kucich U, Fein A, et al. Neutrophil elastase-releasing factors in bronchoalveolar lavage from patients with adult respiratory distress syndrome. Am Rev Respir Dis 1985; 132: 1098–1105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

