Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia
- PMID: 27655849
- PMCID: PMC5436500
- DOI: 10.1126/scitranslmed.aaf2341
Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia
Abstract
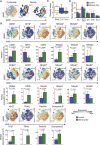
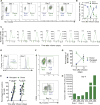
Natural killer (NK) cells are an emerging cellular immunotherapy for patients with acute myeloid leukemia (AML); however, the best approach to maximize NK cell antileukemia potential is unclear. Cytokine-induced memory-like NK cells differentiate after a brief preactivation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine or activating receptor restimulation for weeks to months after preactivation. We hypothesized that memory-like NK cells exhibit enhanced antileukemia functionality. We demonstrated that human memory-like NK cells have enhanced interferon-γ production and cytotoxicity against leukemia cell lines or primary human AML blasts in vitro. Using mass cytometry, we found that memory-like NK cell functional responses were triggered against primary AML blasts, regardless of killer cell immunoglobulin-like receptor (KIR) to KIR-ligand interactions. In addition, multidimensional analyses identified distinct phenotypes of control and memory-like NK cells from the same individuals. Human memory-like NK cells xenografted into mice substantially reduced AML burden in vivo and improved overall survival. In the context of a first-in-human phase 1 clinical trial, adoptively transferred memory-like NK cells proliferated and expanded in AML patients and demonstrated robust responses against leukemia targets. Clinical responses were observed in five of nine evaluable patients, including four complete remissions. Thus, harnessing cytokine-induced memory-like NK cell responses represents a promising translational immunotherapy approach for patients with AML.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures




References
-
- Juliusson G, Antunovic P, Derolf Å, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. - PubMed
-
- Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. - PubMed
-
- Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. - PubMed
-
- Döhner H, Estey E, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. - PubMed
-
- Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, Metzeler KH, Schwind S, Wu Y-Z, Kohlschmidt J, Pettenati MJ, Heerema NA, Block AW, Patil SR, Baer MR, Kolitz JE, Moore JO, Carroll AJ, Stone RM, Larson RA, Bloomfield CD. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

