Actin-based motility of bacterial pathogens: mechanistic diversity and its impact on virulence
- PMID: 27655913
- PMCID: PMC5968334
- DOI: 10.1093/femspd/ftw099
Actin-based motility of bacterial pathogens: mechanistic diversity and its impact on virulence
Abstract
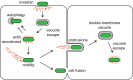
A diverse spectrum of intracellular bacterial pathogens that inhabit the cytosol have evolved the ability to polymerize actin on their surface to power intracellular actin-based motility (ABM). These include species of Listeria, Burkholderia and Rickettsia, as well as Shigella and Mycobacteria Here, we provide an overview of the roles of bacterial ABM in survival and virulence. Moreover, we survey the molecular mechanisms of actin polymerization in host cells and describe how bacterial pathogens mimic or harness the full diversity of these mechanisms for ABM. Finally, we present ABM through a new lens by comparing motility mechanisms between related species of Listeria, Burkholderia, and Rickettsia Through these comparisons, we hope to illuminate how exploitation of different actin polymerization mechanisms influences ABM as well as pathogenicity and virulence in humans and other animals.
Keywords: Bacterial pathogen; Burkholderia; Listeria; Rickettsia; actin-based motility; cytoskeleton.
© FEMS 2016. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Auerbuch V, Loureiro JJ, Gertler FB, et al. Ena/VASP proteins contribute to Listeria monocytogenes pathogenesis by controlling temporal and spatial persistence of bacterial actin-based motility. Mol Microbiol. 2003;49:1361–75. - PubMed
-
- Avvaru BS, Pernier J, Carlier MF. Dimeric WH2 repeats of VopF sequester actin monomers into non-nucleating linear string conformations: an X-ray scattering study. J Struct Biol. 2015;190:192–9. - PubMed
-
- Bachmann C, Fischer L, Walter U, et al. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–57. - PubMed
-
- Bakardjiev AI, Stacy BA, Portnoy DA. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis. 2005;191:1889–97. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

