Eye Can Hear Clearly Now: Inverse Effectiveness in Natural Audiovisual Speech Processing Relies on Long-Term Crossmodal Temporal Integration
- PMID: 27656026
- PMCID: PMC6705572
- DOI: 10.1523/JNEUROSCI.1396-16.2016
Eye Can Hear Clearly Now: Inverse Effectiveness in Natural Audiovisual Speech Processing Relies on Long-Term Crossmodal Temporal Integration
Abstract
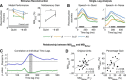
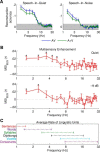
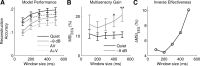
Speech comprehension is improved by viewing a speaker's face, especially in adverse hearing conditions, a principle known as inverse effectiveness. However, the neural mechanisms that help to optimize how we integrate auditory and visual speech in such suboptimal conversational environments are not yet fully understood. Using human EEG recordings, we examined how visual speech enhances the cortical representation of auditory speech at a signal-to-noise ratio that maximized the perceptual benefit conferred by multisensory processing relative to unisensory processing. We found that the influence of visual input on the neural tracking of the audio speech signal was significantly greater in noisy than in quiet listening conditions, consistent with the principle of inverse effectiveness. Although envelope tracking during audio-only speech was greatly reduced by background noise at an early processing stage, it was markedly restored by the addition of visual speech input. In background noise, multisensory integration occurred at much lower frequencies and was shown to predict the multisensory gain in behavioral performance at a time lag of ∼250 ms. Critically, we demonstrated that inverse effectiveness, in the context of natural audiovisual (AV) speech processing, relies on crossmodal integration over long temporal windows. Our findings suggest that disparate integration mechanisms contribute to the efficient processing of AV speech in background noise.
Significance statement: The behavioral benefit of seeing a speaker's face during conversation is especially pronounced in challenging listening environments. However, the neural mechanisms underlying this phenomenon, known as inverse effectiveness, have not yet been established. Here, we examine this in the human brain using natural speech-in-noise stimuli that were designed specifically to maximize the behavioral benefit of audiovisual (AV) speech. We find that this benefit arises from our ability to integrate multimodal information over longer periods of time. Our data also suggest that the addition of visual speech restores early tracking of the acoustic speech signal during excessive background noise. These findings support and extend current mechanistic perspectives on AV speech perception.
Keywords: EEG; envelope tracking; multisensory integration; speech intelligibility; speech-in-noise; stimulus reconstruction.
Copyright © 2016 the authors 0270-6474/16/369888-08$15.00/0.
Figures

References
-
- Bernstein LE, Auer ET, Jr, Takayanagi S. Auditory speech detection in noise enhanced by lipreading. Speech Communication. 2004;44:5–18. doi: 10.1016/j.specom.2004.10.011. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
