Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala
- PMID: 27656031
- PMCID: PMC5030354
- DOI: 10.1523/JNEUROSCI.0953-16.2016
Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala
Abstract
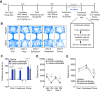
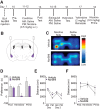
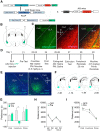
The dynorphin (DYN)/kappa-opioid receptor (KOR) system plays a conserved role in stress-induced reinstatement of drug seeking for prototypical substances of abuse. Due to nicotine's high propensity for stress-induced relapse, we hypothesized that stress would induce reinstatement of nicotine seeking-like behavior in a KOR-dependent manner. Using a conditioned place preference (CPP) reinstatement procedure in mice, we show that both foot-shock stress and the pharmacological stressor yohimbine (2 mg/kg, i.p.) induce reinstatement of nicotine CPP in a norbinaltorphimine (norBNI, a KOR antagonist)-sensitive manner, indicating that KOR activity is necessary for stress-induced nicotine CPP reinstatement. After reinstatement testing, we visualized robust c-fos expression in the basolateral amygdala (BLA), which was reduced in mice pretreated with norBNI. We then used several distinct but complementary approaches of locally disrupting BLA KOR activity to assess the role of KORs and KOR-coupled intracellular signaling cascades on reinstatement of nicotine CPP. norBNI injected locally into the BLA prevented yohimbine-induced nicotine CPP reinstatement without affecting CPP acquisition. Similarly, selective deletion of BLA KORs in KOR conditional knock-out mice prevented foot-shock-induced CPP reinstatement. Together, these findings strongly implicate BLA KORs in stress-induced nicotine seeking-like behavior. In addition, we found that chemogenetic activation of Gαi signaling within CaMKIIα BLA neurons was sufficient to induce nicotine CPP reinstatement, identifying an anatomically specific intracellular mechanism by which stress leads to reinstatement. Considered together, our findings suggest that activation of the DYN/KOR system and Gαi signaling within the BLA is both necessary and sufficient to produce reinstatement of nicotine preference.
Significance statement: Considering the major impact of nicotine use on human health, understanding the mechanisms by which stress triggers reinstatement of drug-seeking behaviors is particularly pertinent to nicotine. The dynorphin (DYN)/kappa-opioid receptor (KOR) system has been implicated in stress-induced reinstatement of drug seeking for other commonly abused drugs. However, the specific role, brain region, and mechanisms that this system plays in reinstatement of nicotine seeking has not been characterized. Here, we report region-specific engagement of the DYN/KOR system and subsequent activation of inhibitory (Gi-linked) intracellular signaling pathways within the basolateral amygdala during stress-induced reinstatement of nicotine preference. We show that the DYN/KOR system is necessary to produce this behavioral state. This work may provide novel insight for the development of therapeutic approaches to prevent stress-related nicotine relapse.
Keywords: basolateral amygdala; conditioned place preference; kappa opioid receptors; nicotine; reinstatement; stress.
Copyright © 2016 the authors 0270-6474/16/369938-12$15.00/0.
Figures



Similar articles
-
Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism.Neuropsychopharmacology. 2013 Nov;38(12):2484-97. doi: 10.1038/npp.2013.151. Epub 2013 Jun 21. Neuropsychopharmacology. 2013. PMID: 23787819 Free PMC article.
-
Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice.Psychopharmacology (Berl). 2013 Apr;226(4):763-8. doi: 10.1007/s00213-012-2716-y. Epub 2012 Apr 20. Psychopharmacology (Berl). 2013. PMID: 22526543 Free PMC article.
-
Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats.Behav Brain Res. 2014 May 15;265:188-97. doi: 10.1016/j.bbr.2014.02.029. Epub 2014 Feb 28. Behav Brain Res. 2014. PMID: 24583188 Free PMC article.
-
Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: Pharmacological effects and addiction in animal models and humans.Eur J Neurosci. 2019 Aug;50(3):2247-2254. doi: 10.1111/ejn.13970. Epub 2018 Aug 31. Eur J Neurosci. 2019. PMID: 29802666 Review.
-
Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol.Alcohol Clin Exp Res. 2017 Aug;41(8):1402-1418. doi: 10.1111/acer.13406. Epub 2017 Jun 5. Alcohol Clin Exp Res. 2017. PMID: 28425121 Free PMC article. Review.
Cited by
-
Dopamine D3 Receptor Modulates Akt/mTOR and ERK1/2 Pathways Differently during the Reinstatement of Cocaine-Seeking Behavior Induced by Psychological versus Physiological Stress.Int J Mol Sci. 2023 Jul 7;24(13):11214. doi: 10.3390/ijms241311214. Int J Mol Sci. 2023. PMID: 37446391 Free PMC article.
-
Imaging Kappa Opioid Receptors in the Living Brain with Positron Emission Tomography.Handb Exp Pharmacol. 2022;271:547-577. doi: 10.1007/164_2021_498. Handb Exp Pharmacol. 2022. PMID: 34363128 Review.
-
Modulation of Nicotine-Associated Behaviour in Rats By μ-Opioid Signals from the Medial Prefrontal Cortex to the Nucleus Accumbens Shell.Neurosci Bull. 2024 Dec;40(12):1826-1842. doi: 10.1007/s12264-024-01230-1. Epub 2024 Jun 8. Neurosci Bull. 2024. PMID: 38850386
-
Altered mRNA Levels of Stress-Related Peptides in Mouse Hippocampus and Caudate-Putamen in Withdrawal after Long-Term Intermittent Exposure to Tobacco Smoke or Electronic Cigarette Vapour.Int J Mol Sci. 2021 Jan 9;22(2):599. doi: 10.3390/ijms22020599. Int J Mol Sci. 2021. PMID: 33435320 Free PMC article.
-
Formation and fate of an engram in the lateral amygdala supporting a rewarding memory in mice.Neuropsychopharmacology. 2023 Apr;48(5):724-733. doi: 10.1038/s41386-022-01472-5. Epub 2022 Oct 19. Neuropsychopharmacology. 2023. PMID: 36261624 Free PMC article.
References
-
- al'Absi M. Stress and addiction: Biological and psychosocial mechanisms. London: Elsevier Academic Press; 2007.
-
- Al-Hasani R, McCall JG, Shin G, Gomez A.M., Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–1077. doi: 10.1016/j.neuron.2015.08.019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
