The Wiring of Developing Sensory Circuits-From Patterned Spontaneous Activity to Synaptic Plasticity Mechanisms
- PMID: 27656131
- PMCID: PMC5011135
- DOI: 10.3389/fncir.2016.00071
The Wiring of Developing Sensory Circuits-From Patterned Spontaneous Activity to Synaptic Plasticity Mechanisms
Abstract
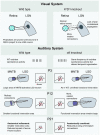
In order to accurately process incoming sensory stimuli, neurons must be organized into functional networks, with both genetic and environmental factors influencing the precise arrangement of connections between cells. Teasing apart the relative contributions of molecular guidance cues, spontaneous activity and visual experience during this maturation is on-going. During development of the sensory system, the first, rough organization of connections is created by molecular factors. These connections are then modulated by the intrinsically generated activity of neurons, even before the senses have become operational. Spontaneous waves of depolarizations sweep across the nervous system, placing them in a prime position to strengthen correct connections and weaken others, shaping synapses into a useful network. A large body of work now support the idea that, rather than being a mere side-effect of the system, spontaneous activity actually contains information which readies the nervous system so that, as soon as the senses become active, sensory information can be utilized by the animal. An example is the neonatal mouse. As soon as the eyelids first open, neurons in the cortex respond to visual information without the animal having previously encountered structured sensory input (Cang et al., 2005b; Rochefort et al., 2011; Zhang et al., 2012; Ko et al., 2013). In vivo imaging techniques have advanced considerably, allowing observation of the natural activity in the brain of living animals down to the level of the individual synapse. New (opto)genetic methods make it possible to subtly modulate the spatio-temporal properties of activity, aiding our understanding of how these characteristics relate to the function of spontaneous activity. Such experiments have had a huge impact on our knowledge by permitting direct testing of ideas about the plasticity mechanisms at play in the intact system, opening up a provocative range of fresh questions. Here, we intend to outline the most recent descriptions of spontaneous activity patterns in rodent developing sensory areas, as well as the inferences we can make about the information content of those activity patterns and ideas about the plasticity rules that allow this activity to shape the young brain.
Keywords: auditory system development; developmental biology; plasticity mechanisms; spontaneous activity; synaptic plasticity; visual system development.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

