The Nucleotide Excision Repair Pathway Protects Borrelia burgdorferi from Nitrosative Stress in Ixodes scapularis Ticks
- PMID: 27656169
- PMCID: PMC5013056
- DOI: 10.3389/fmicb.2016.01397
The Nucleotide Excision Repair Pathway Protects Borrelia burgdorferi from Nitrosative Stress in Ixodes scapularis Ticks
Abstract
The Lyme disease spirochete Borrelia burgdorferi encounters a wide range of environmental conditions as it cycles between ticks of the genus Ixodes and its various mammalian hosts. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are potent antimicrobial molecules generated during the innate immune response to infection, however, it is unclear whether ROS and RNS pose a significant challenge to B. burgdorferi in vivo. In this study, we screened a library of B. burgdorferi strains with mutations in DNA repair genes for increased susceptibility to ROS or RNS in vitro. Strains with mutations in the methyl-directed mismatch repair gene mutS1 are hypersensitive to killing by ROS, while strains lacking the nucleotide excision repair (NER) gene uvrB show increased susceptibility to both ROS and RNS. Therefore, mutS1-deficient and uvrB-deficient strains were compared for their ability to complete their infectious cycle in Swiss Webster mice and I. scapularis ticks to help identify sites of oxidative and nitrosative stresses encountered by B. burgdorferi in vivo. Both mutS1 and uvrB were dispensable for infection of mice, while uvrB promoted the survival of spirochetes in I. scapularis ticks. The decreased survival of uvrB-deficient B. burgdorferi was associated with the generation of RNS in I. scapularis midguts and salivary glands during feeding. Collectively, these data suggest that B. burgdorferi must withstand cytotoxic levels of RNS produced during infection of I. scapularis ticks.
Keywords: Borrelia; DNA repair; Lyme disease; nitric oxide; oxidative stress.
Figures
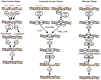



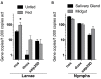
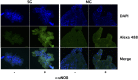

References
-
- Adams D. A., Gallagher K. M., Jajosky R. A., Kriseman J., Sharp P., Anderson W. J., et al. (2013). Summary of notifiable diseases - United States, 2011. MMWR Morb. Mortal. Wkly. Rep. 60 1–117. - PubMed
-
- Araujo R. N., Soares A. C., Paim R. M., Gontijo N. F., Gontijo A. F., Lehane M. J., et al. (2009). The role of salivary nitrophorins in the ingestion of blood by the triatomine bug Rhodnius prolixus (Reduviidae: Triatominae). Insect Biochem. Mol. Biol. 39 83–89. 10.1016/j.ibmb.2008.10.002 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

